
Concept explainers
Identify the following bases, and tell whether each is found in DNA, RNA, or both:
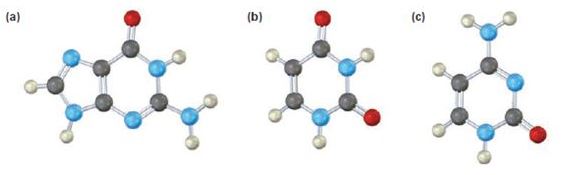
a)
Interpretation:
To identify the following bases and state whether each lie in DNA, RNA or both.
Concept introduction:
Proteins and biopolymers are made up of amino acids, nucleic acids which are joined together to form a long chain. Nucleic acids are the biopolymers of nucleotides, which is composed of a nucleoside. Each nucleoside is bonded to a phosphate group.
Each nucleoside is composed of an aldopentose sugar linked through its anomeric carbon to the nitrogen atom of a heterocyclic purine or pyrimidine base. RNA contains ribose as a sugar component and DNA contains 29-deoxyribose as a sugar component.
DNA contains four different amine bases: two substituted purines (adenine and guanine) and two substituted pyrimidines (cytosine and thymine). Adenine, guanine, and cytosine also occur in RNA, but thymine is replaced in RNA by a closely related pyrimidine base called uracil.
Answer to Problem 13VC
The base given here is: Guanine (G)
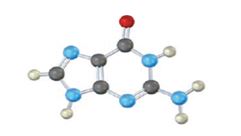
It is found in both DNA and RNA.
Explanation of Solution
In the above diagrams of bases, guanine and cytosine are found in DNA and RNA. Whereas, uracil is found in only RNA. Here the blue atoms are the symbols for nitrogen, red atom represents oxygen, black atom represents carbon and grey atom represnts hydrogen.
The base given here is: Guanine (G)

It is found in both DNA and RNA.
b)
Interpretation:
To identify the following bases and state whether each lie in DNA, RNA or both.
Concept introduction:
Proteins and biopolymers are made up of amino acids, nucleic acids which are joined together to form a long chain. Nucleic acids are the biopolymers of nucleotides, which is composed of a nucleoside. Each nucleoside is bonded to a phosphate group.
Each nucleoside is composed of an aldopentose sugar linked through its anomeric carbon to the nitrogen atom of a heterocyclic purine or pyrimidine base. RNA contains ribose as a sugar component and DNA contains 29-deoxyribose as a sugar component.
DNA contains four different amine bases: two substituted purines (adenine and guanine) and two substituted pyrimidines (cytosine and thymine). Adenine, guanine, and cytosine also occur in RNA, but thymine is replaced in RNA by a closely related pyrimidine base called uracil.
Answer to Problem 13VC
The base given here is: Uracil (U)
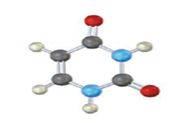
It is found in RNA.
Explanation of Solution
In the above diagrams of bases, guanine and cytosine are found in DNA and RNA. Whereas, uracil is found in only RNA. Here the blue atoms are the symbols for nitrogen, red atom represents oxygen, black atom represents carbon and grey atom represnts hydrogen.
The base given here is: Uracil (U)

It is found in RNA.
c)
Interpretation:
To identify the following bases and state whether each lie in DNA, RNA or both.
Concept introduction:
Proteins and biopolymers are made up of amino acids, nucleic acids which are joined together to form a long chain. Nucleic acids are the biopolymers of nucleotides, which is composed of a nucleoside. Each nucleoside is bonded to a phosphate group.
Each nucleoside is composed of an aldopentose sugar linked through its anomeric carbon to the nitrogen atom of a heterocyclic purine or pyrimidine base. RNA contains ribose as a sugar component and DNA contains 29-deoxyribose as a sugar component.
DNA contains four different amine bases: two substituted purines (adenine and guanine) and two substituted pyrimidines (cytosine and thymine). Adenine, guanine, and cytosine also occur in RNA, but thymine is replaced in RNA by a closely related pyrimidine base called uracil.
Answer to Problem 13VC
The base given here is: Cytosine (C)
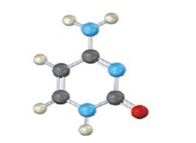
It is found in both DNA and RNA.
Explanation of Solution
In the above diagrams of bases, guanine and cytosine are found in DNA and RNA. Whereas, uracil is found in only RNA. Here the blue atoms are the symbols for nitrogen, red atom represents oxygen, black atom represents carbon and grey atom represnts hydrogen.
The base given here is: Cytosine (C)

It is found in both DNA and RNA.
Want to see more full solutions like this?
Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
Organic Chemistry
General, Organic, and Biological Chemistry - 4th edition
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
The Cosmic Perspective (8th Edition)
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





