
(a)
Interpretation: MOs for the given conjugated systems has to be drawn and the HOMO has to be predicted as symmetric or antisymmetric.
Concept introduction:
Conjugated system: A system of connected p-orbitals with delocalized electrons with alternating single and multiple bonds and the compound may be cyclic, linear or mixed.
Molecular orbital theory suggests that atomic orbitals of different atoms combines to create molecular orbitals.
Molecular orbitals can be constructed from linear combination of atomic orbitals.
Bonding orbotals are formed by the additive combination of atomic orbitals and the antibonding orbitals are formed by the substractive combination of atomic orbitals.
Antibonding orbital is a molecular orbital that results when two parallel atomic orbitals with opposite phases interact.
Antibonding orbitals have higher energy than the bonding molecular orbitals.
Ground state and and exited states are the positions with lower and higher energy respectively.
HOMO is a molecular orbital which is the abbrevation of Highest Occupied Molecular Orbital.
LUMO is also a molecular orbital which is the short form of Lowest Unoccupied Molecular Orbital.
If the lobes at the ends of the MO are in phase, then the MO is symmetric.
If the two lobes are out phase then the MO is antisymmetric.
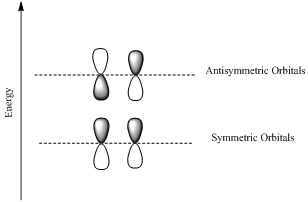
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
(b)
Interpretation: The validity of Woodward – Hoffmann rule for an electrocyclic reaction has to be checked using the MOs of given systems.
Concept introduction:
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
EBK ORGANIC CHEMISTRY
- What will the enolate for this be using LDA, THF, and cold temperatures? What will it be using NaOEt at rt?arrow_forwardHelp me solve this problem.arrow_forwardDraw a mechanism for the following synthetic transformation including reagents and any isolable intermediates throughout the process. Please clearly indicate bond cleavage/formation using curly arrows. MeO2Carrow_forward
- CHEM 310 Quiz 8 Organic Chemistry II Due: Tuesday, April 25th, at 11:59 pm. This quiz is open textbook / open notes - but you must work alone. You cannot use the internet or the solutions manual for the book. Scan in your work and record an explanation of your mechanism. You may record this any way that you like. One way would be to start an individual Zoom meeting, start recording, "share your screen" and then talk through the problem. This will be converted to an .mp4 file that you can upload into Canvas using the "record/upload media" feature. Pyridine, benzoic acid and benzene are dissolved in ethyl acetate. Design and provide a plan / flow chart for separating and isolating each of these components. Pyridine and benzene are liquids at room temperature. Benzoic acid is a solid. You have ethyl acetate, 2M NaOH, 2M HCI and anhydrous MgSO4 available, as well as all the glassware and equipment that you used in the organic lab this year. Provide accurate acid/base reactions for any…arrow_forwardCan anyone help me solve this step by step. Thank you in advaarrow_forwardPlease draw the mechanism for this Friedel-crafts acylation reaction using arrowsarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
