
(a)
Interpretation:
The resonance contributors for given species has to be drawn.
Concept Introduction:
Localized electrons:
If the negative charge formed by losing a proton resides only on one atom, they are termed as localized electrons. For example, if an alcohol loses a proton, the electrons remaining will be resides on its single oxygen atom.
Delocalized electrons:
If the negative charge formed by losing a proton resides on more than two atoms, they are termed as delocalized electrons. For example, if
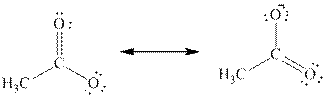
The above two structures are known as resonance contributors and the actual structure which is called the resonance hybrid will be the composite of the two resonance contributors.
(b)
Interpretation:
The resonance contributors for given species has to be drawn.
Concept Introduction:
Localized electrons:
If the negative charge formed by losing a proton resides only on one atom, they are termed as localized electrons. For example, if an alcohol loses a proton, the electrons remaining will be resides on its single oxygen atom.
Delocalized electrons:
If the negative charge formed by losing a proton resides on more than two atoms, they are termed as delocalized electrons. For example, if carboxylic acid loses a proton, the electrons remaining will be resides on both oxygen atoms and thus reduces the electron density of the atom making the conjugate base more stable.
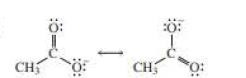
The above two structures are known as resonance contributors and the actual structure which is called the resonance hybrid will be the composite of the two resonance contributors.
Trending nowThis is a popular solution!

Chapter 2 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- Please correct answer and don't use hand ratingarrow_forwardSafari File Edit View History Bookmarks Window Help く < mylabmastering.pearson.com Wed Feb 12 8:44 PM ✩ + Apple Q Bing Google SignOutOptions M Question 36 - Lab HW BI... P Pearson MyLab and Mast... P Course Home Error | bartleby b Answered: If the biosynth... Draw a free-radical mechanism for the following reaction, forming the major monobromination product: ScreenPal - 2022 CHEM2... Access Pearson 2 CH3 Br-Br CH H3 Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron- flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. ▸ View Available Hint(s) 0 2 DE [1] H EXP. CONT. H. Br-Br H FEB 12arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forwardQ1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. + CI Br : Н OH H wo་ཡིག་ཐrow HO 3 D ။။ဂ CI Br H, CI Br Br H₂N OMe R IN I I N S H Br ជ័យ CI CI D OHarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forward%Reflectance 95 90- 85 22 00 89 60 55 50 70 65 75 80 50- 45 40 WA 35 30- 25 20- 4000 3500 Date: Thu Feb 06 17:21:21 2025 (GMT-05:0(UnknownD Scans: 8 Resolution: 2.000 3000 2500 Wavenumbers (cm-1) 100- 2981.77 1734.25 2000 1500 1000 1372.09 1108.01 2359.09 1469.82 1181.94 1145.20 1017.01 958.45 886.97 820.49 668.25 630.05 611.37arrow_forwardNonearrow_forwardCH3 CH H3C CH3 H OH H3C- -OCH2CH3 H3C H -OCH3 For each of the above compounds, do the following: 1. List the wave numbers of all the IR bands in the 1350-4000 cm-1 region. For each one, state what bond or group it represents. 2. Label equivalent sets of protons with lower-case letters. Then, for each 1H NMR signal, give the 8 value, the type of splitting (singlet, doublet etc.), and the number protons it represents. of letter δ value splitting # of protons 3. Redraw the compound and label equivalent sets of carbons with lower-case letters. Then for each set of carbons give the 5 value and # of carbons it represents. letter δ value # of carbonsarrow_forwardDraw the correct ionic form(s) of arginine at the pKa and PI in your titration curve. Use your titration curve to help you determine which form(s) to draw out.arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



