
(a)
Interpretation:
The resonance contributors for given species has to be drawn.
Concept Introduction:
Localized electrons:
If the negative charge formed by losing a proton resides only on one atom, they are termed as localized electrons. For example, if an alcohol loses a proton, the electrons remaining will be resides on its single oxygen atom.
Delocalized electrons:
If the negative charge formed by losing a proton resides on more than two atoms, they are termed as delocalized electrons. For example, if
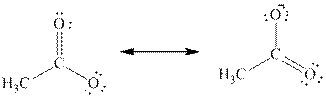
The above two structures are known as resonance contributors and the actual structure which is called the resonance hybrid will be the composite of the two resonance contributors.
(b)
Interpretation:
The resonance contributors for given species has to be drawn.
Concept Introduction:
Localized electrons:
If the negative charge formed by losing a proton resides only on one atom, they are termed as localized electrons. For example, if an alcohol loses a proton, the electrons remaining will be resides on its single oxygen atom.
Delocalized electrons:
If the negative charge formed by losing a proton resides on more than two atoms, they are termed as delocalized electrons. For example, if carboxylic acid loses a proton, the electrons remaining will be resides on both oxygen atoms and thus reduces the electron density of the atom making the conjugate base more stable.
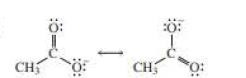
The above two structures are known as resonance contributors and the actual structure which is called the resonance hybrid will be the composite of the two resonance contributors.
Trending nowThis is a popular solution!

Chapter 2 Solutions
ORGANIC CHEMISTRY-W/S.G+SOLN.MANUAL
- Draw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forwardURGENT! PLEASE HELP!arrow_forward
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forwardCan you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



