
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
8th Edition
ISBN: 9781337687539
Author: Brown/Iverson/Anslyn/ Foote
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 28, Problem 28.7P
Interpretation Introduction
Interpretation:
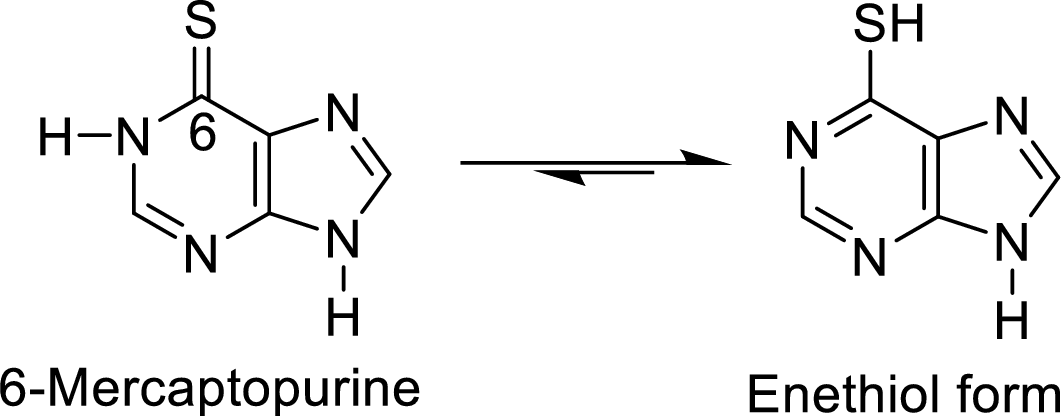
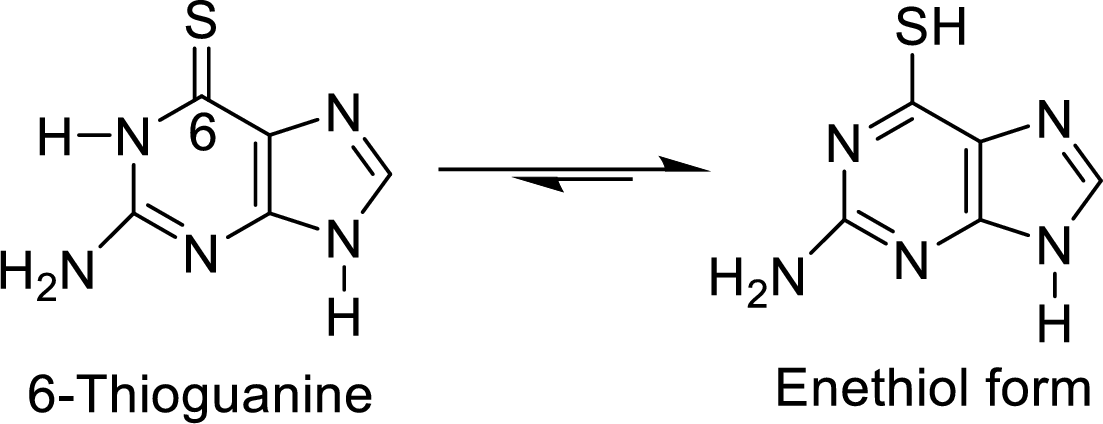
The structural formulas for the enethiol forms of 6-mercaptopurine and 6-thioguanine has to be drawn.
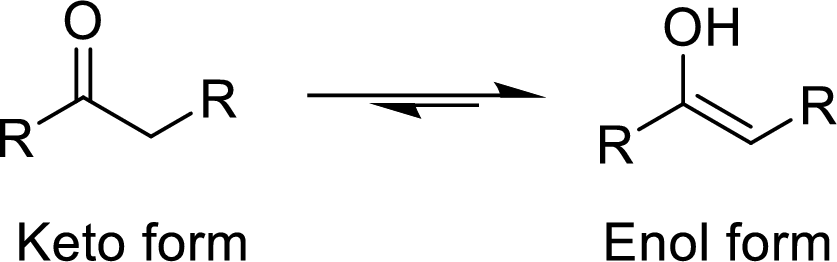
Concept Introduction:
The
Expert Solution & Answer
Explanation of Solution
The enethiol is also the enol form which contains Sulphur molecule instead of Oxygen. The enethiol form is the alcoholic form of the Sulphur. The double bonded Sulphur is converted to thiol form of the Sulphur.
The enethiol form of 6-mercaptopurine is
The enethiol form of 6-thioguanine is
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
>
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other
major side products:
1. ☑
CI
2. H3O+
O
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the
drawing area and leave it blank.
Click and drag to start drawing a
structure.
Explanation
Check
?
DO
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Don't use ai to answer I will report you answer
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
Chapter 28 Solutions
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
Ch. 28.1 - Prob. 28.1PCh. 28.2 - Prob. 28.2PCh. 28.2 - Prob. 28.3PCh. 28.3 - Here is a portion of the nucleotide sequence in...Ch. 28.4 - The following section of DNA codes for oxytocin, a...Ch. 28.5 - Prob. 28.6PCh. 28 - Prob. 28.7PCh. 28 - Following are structural formulas for cytosine and...Ch. 28 - Prob. 28.9PCh. 28 - Prob. 28.10P
Ch. 28 - Prob. 28.11PCh. 28 - Prob. 28.12PCh. 28 - Prob. 28.13PCh. 28 - Prob. 28.14PCh. 28 - Prob. 28.15PCh. 28 - Draw a structural formula of the DNA...Ch. 28 - List the postulates of the Watson-Crick model of...Ch. 28 - Prob. 28.18PCh. 28 - Prob. 28.19PCh. 28 - Prob. 28.20PCh. 28 - Prob. 28.21PCh. 28 - Prob. 28.22PCh. 28 - Prob. 28.23PCh. 28 - Prob. 28.24PCh. 28 - Write the DNA complement for 5-ACCGTTAAT-3. Be...Ch. 28 - Prob. 28.26PCh. 28 - Prob. 28.27PCh. 28 - Compare DNA and RNA is these ways. (a)...Ch. 28 - What type of RNA has the shortest lifetime in...Ch. 28 - Prob. 28.30PCh. 28 - Prob. 28.31PCh. 28 - Prob. 28.32PCh. 28 - Write the mRNA codons for the following. (a)...Ch. 28 - Prob. 28.34PCh. 28 - Prob. 28.35PCh. 28 - Prob. 28.36PCh. 28 - Prob. 28.37PCh. 28 - Prob. 28.38PCh. 28 - Prob. 28.39PCh. 28 - What polypeptide is coded for by this mRNA...Ch. 28 - The alpha chain of human hemoglobin has 141 amino...Ch. 28 - Prob. 28.42P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY