
Concept explainers
(a)
Interpretation:
The hydrocarbon B and the intermediate A (both with the formulas C11H10) in the following reaction sequence is to be identified. Compound B is formed spontaneously from A in a pericyclic reaction.
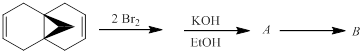
Concept introduction:
Commonly, electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner.
(b)
Interpretation:
The proton NMR spectrum of B consists of a complex absorption at δ 7.1 (8H) and a singlet at δ (-0.5) (2H).The negative absorption for protons is to be explained.
Concept introduction:
The usage of nuclear magnetic resonance in NMR spectroscopy of hydron nuclei is termed as proton nuclear magnetic resonance spectroscopy. This is used to determine the structure of molecules with respect to 1H found within the molecules. In order to avoid the interference from solvent molecules, the analysis of 1H spectra is usually carried out in deuterated solvents such as D2O, CDCl3, DMSO-d6.Besides, tetramethyl silane is used as an internal standard.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- a. Determine whether each of the Followery Molecules is in the R- On the y- Configuration 1-01"/ 1-6-4 Br 4 I el Br b. Draw The Fisher projection For all the Meso compounds that can exist FOR The Following molenlearrow_forward1- Refer to the monosaccharides below to answer each of the following question(s): CH₂OH CHO CH₂OH CH₂OH 0 H- OH 0 0 HO- H H- -OH HO H HO H H OH HO- H CH₂OH H. OH HO H HO- H CH₂OH CH₂OH CH3 a. Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. a. Xylulose is .. b. Erythrulose is . c. Sorbose is .. d. Rhamnose is .. 2- Consider the reaction below to answer the following question(s). CHO H OH CH₂OH CH₂OH HO- H HO HO + H. -OH HO OH HO. H OH OH H -OH H OH CH₂OH Q Z a. Refer to Exhibit 25-11. Place a triangle around the anomeric carbon in compound Q. Compound Z is: b. 1. the D-anomer. 2. the a-anomer. 3. the ẞ-anomer. 4. the L-anomer. c. Which anomer is the LEAST stable? d. Q and Z are cyclic examples of: a. acetals b. hemiacetals c. alditols d. hemialditolsarrow_forwardi need help identifying the four carbon oxygen bonds in the following:arrow_forward
- Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule HO H3N + The solution is... X O acidic OH O basic H3N-CH-C-O O neutral ○ (unknown) O acidic ○ basic CH2 CH 3-S-CH2 O neutral ○ (unknown) H3N O OH O acidic O basic Oneutral O (unknown) 0 H3N-CH-C-O CH3 CH CH3 O acidic O basic O neutral ○ (unknown) ? olo Ar BHarrow_forwardno Ai walkthroughs need other product (product in picture is wrong dont submit the same thing)arrow_forwardHow to solve this!arrow_forward
- I have a 2 mil plastic film that degrades in 22 days at 88C and 153 days at 61C what is the predicted theoretical degradation at 47C?arrow_forwardno ai walkthrougharrow_forwardI have a 2 mil plastic film that degrades after 22 days at 88C and at 61C takes 153 days. What is the failure at 47C in days.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

