
EBK PHYSICS
5th Edition
ISBN: 9780134051796
Author: Walker
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 79PCE
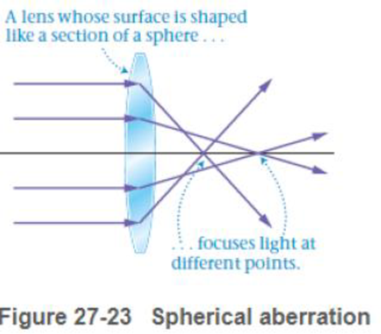
The focal length for light that strikes near the center of a spherical convex lens is 15 cm. Referring to Figure 27-23,will the focal length for light that strikes near the edge of the lens be greater than: less than; or equal to 15 cm?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).
What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V)
ammeter
I =
simple diagram to illustrate the setup for each law- coulombs law and biot savart law
Chapter 27 Solutions
EBK PHYSICS
Ch. 27.1 - If the f -number on a camera is increased does the...Ch. 27.2 - Prob. 2EYUCh. 27.3 - A magnifying glass is held over a ruled piece of...Ch. 27.4 - Rank the following microscopes in order of...Ch. 27.5 - In a typical telescope, is foppose greater than,...Ch. 27.6 - One advantage of reflecting telescopes over...Ch. 27 - Prob. 1CQCh. 27 - Prob. 2CQCh. 27 - If your near-point distance is N, how close can...Ch. 27 - When you open your eyes underwater, everything...
Ch. 27 - When you use a simple magnifying glass, does it...Ch. 27 - Does chromatic aberration occur in mirrors?...Ch. 27 - BIO Predict/Explain Octopus Eyes To focus its...Ch. 27 - Your friend is 1.7 m tall. (a) When she stands 3.2...Ch. 27 - Which forms the larger image on the retina of your...Ch. 27 - Approximating the eye as a single thin lens 2.70...Ch. 27 - Approximating the eye as a single thin lens 2.70...Ch. 27 - Find the far-point distance of a person whose...Ch. 27 - Four camera lenses have the following focal...Ch. 27 - BIO The focal length of the human eye is...Ch. 27 - Predict/Calculate A camera with a...Ch. 27 - The actual light sensor size of a digital camera...Ch. 27 - (a) Find the f -number of a telescope with an...Ch. 27 - You are taking a photo of a poster on the wall of...Ch. 27 - You are taking pictures of the beach at sunset...Ch. 27 - Predict/Calculate You are taking a photograph of a...Ch. 27 - The Hale Telescope The 200-in. (5.08-m) diameter...Ch. 27 - Predict/Explain Two professors are stranded on a...Ch. 27 - A clerk at the local grocery store wears glasses...Ch. 27 - The umpire at a baseball game wears glasses that...Ch. 27 - A police detective discovers eyeglasses with a...Ch. 27 - BIO The cornea of a normal human eye has an...Ch. 27 - A myopic student is shaving without his glasses....Ch. 27 - An eyeglass prescription calls for a lens with an...Ch. 27 - An optometrist prescribes contact lenses with a...Ch. 27 - Two thin lenses, with f1 = +25.0 cm and f2 = 42.5...Ch. 27 - Two concave lenses, each with f = 15 cm, are...Ch. 27 - BIO Predict/Calculate The focal length of a...Ch. 27 - BIO Predict/Calculate Diopter Change in Diving...Ch. 27 - A converging lens of focal length 9,000 cm is 18.0...Ch. 27 - Repeat Problem 28, this time with the coin placed...Ch. 27 - Find the focal length of contact lenses that would...Ch. 27 - Find the focal length of contact lenses that would...Ch. 27 - What focal length should a pair of contact lenses...Ch. 27 - Reading glasses with a power of + 1.50 diopters...Ch. 27 - A nearsighted person wears contacts with a focal...Ch. 27 - Without his glasses, Isaac can see objects clearly...Ch. 27 - A person whose near-point distance is 42.5 cm...Ch. 27 - A pair of eyeglasses is designed to allow a person...Ch. 27 - Predict/Calculate Your favorite aunt can read a...Ch. 27 - Predict/Calculate The relaxed eyes of a patient...Ch. 27 - Without glasses, your Uncle Albert can see things...Ch. 27 - A 2.05-cm-tall object is placed 30.0 cm to the...Ch. 27 - A simple camera telephoto lens consists of two...Ch. 27 - Predict/Calculate With unaided vision, a librarian...Ch. 27 - A persons prescription for her new bifocal glasses...Ch. 27 - A persons prescription for his new bifocal...Ch. 27 - Two lenses, with f1 = +20.0 cm and f2 = +30.0 cm,...Ch. 27 - A converging lens with a focal length of 4.0 cm is...Ch. 27 - Two magnifying glasses are for sale at a store....Ch. 27 - The Moon is 3476 km in diameter and orbits the...Ch. 27 - A magnifying glass is a single convex lens with a...Ch. 27 - Calculate the focal length of a magnifying lens...Ch. 27 - Predict/Calculate A student has two lenses, one of...Ch. 27 - A beetle 4.93 mm long is examined with a simple...Ch. 27 - To engrave wishes of good luck on a watch, an...Ch. 27 - A jeweler examines a diamond with a magnifying...Ch. 27 - In Problem 55, find the angular magnification when...Ch. 27 - Prob. 57PCECh. 27 - You have two lenses: lens 1 with a focal length of...Ch. 27 - Predict/Calculate Microscope objective A is...Ch. 27 - A compound microscope has an objective lens with a...Ch. 27 - BIO A typical red blood cell subtends an angle of...Ch. 27 - (a) If you treat a 10x eyepiece of a microscope as...Ch. 27 - The medium-power objective lens in a laboratory...Ch. 27 - A compound microscope has the objective and...Ch. 27 - The barrel of a compound microscope is 15 cm in...Ch. 27 - A compound microscope uses a 75.0-mm lens as the...Ch. 27 - The tube length of a microscope is defined to be...Ch. 27 - Two telescopes of different lengths produce the...Ch. 27 - A grade school student plans to build a 35-power...Ch. 27 - A 75-power refracting telescope has an eyepiece...Ch. 27 - An amateur astronomer wants to build a small...Ch. 27 - A pirate sights a distant ship with a spyglass...Ch. 27 - A telescope has lenses with focal lengths f1 =...Ch. 27 - Jason has a 25-power telescope whose objective...Ch. 27 - Roughing It with Science A professor shipwrecked...Ch. 27 - Galileos Telescope Galileos first telescope used a...Ch. 27 - The Moon has an angular size of 0 50 when viewed...Ch. 27 - A telescope is 275 mm long and has an objective...Ch. 27 - The focal length for light that strikes near the...Ch. 27 - The focal length for red light that strikes a...Ch. 27 - BIO Predict/Explain Intracorneal Ring An...Ch. 27 - CE BIO The lens in a normal human eye, with...Ch. 27 - CE BIO Predict/Explain Treating Cataracts When the...Ch. 27 - Galileos original telescope (Figure 27-29) used a...Ch. 27 - Predict/Calculate For each of the following cases,...Ch. 27 - Predict/Calculate You have two lenses, with focal...Ch. 27 - BIO The eye is actually a multiple-lens system,...Ch. 27 - BIO Fitting Contact Lenses with a Keratometer When...Ch. 27 - Pricey Stamp A rare 1918 Jenny stamp, depicting a...Ch. 27 - Prob. 90GPCh. 27 - Consider a Galilean telescope, as illustrated in...Ch. 27 - A farsighted person uses glasses with a refractive...Ch. 27 - Landing on an Aircraft Carrier The Fresnel Lens...Ch. 27 - A Cassegrain astronomical telescope uses two...Ch. 27 - Predict/Calculate A convex Ions (f = 20.0 cm) is...Ch. 27 - The diameter of a collimated laser beam can be...Ch. 27 - Consider three lenses with focal lengths of 25.0...Ch. 27 - Because a concave lens cannot form a real image of...Ch. 27 - A person with a near-point distance N uses a...Ch. 27 - Prob. 100GPCh. 27 - Prob. 101PPCh. 27 - Prob. 102PPCh. 27 - Prob. 103PPCh. 27 - Predict/Calculate Referring to Example 27-4...Ch. 27 - Predict/Calculate Referring to Example 27-4 in...Ch. 27 - Predict/Calculate Referring to Example 27-4 In...Ch. 27 - Predict/Calculate Referring to Example 27-6...Ch. 27 - Predict/Calculate Referring to Example 27-6...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
A wild-type fruit fly (heterozygous for gray body color and led eyes) is mated Willi a black fruit fly wltli pu...
Campbell Biology (11th Edition)
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
Which of the following factors would tend to increase membrane fluidity? A. a greater proportion of unsaturated...
Campbell Biology in Focus (2nd Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Why is an endospore called a resting structure? Of what advantage is an endospore to a bacterial cell?
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forwardAn L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forward
- Describe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forwardDiscuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward
- 3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forwardA 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forward
- When violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forwardTwo complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all stepsarrow_forwardCalculate the center of mass of the hollow cone shown below. Clearly specify the origin and the coordinate system you are using. Z r Y h Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Convex and Concave Lenses; Author: Manocha Academy;https://www.youtube.com/watch?v=CJ6aB5ULqa0;License: Standard YouTube License, CC-BY