
Concept explainers
Draw the product formed when the following amino acid is treated with each reagent:
(a)
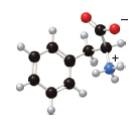
(a)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 27P
The products formed by the treatment of given amino acid with
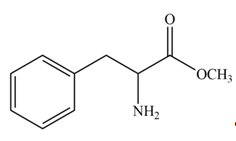
Explanation of Solution
In the given ball-and-stick model of amino acid, the black balls represent carbon atoms, the white balls represent hydrogen atoms, the blue ball represents nitrogen atom and the red balls represent oxygen atoms. Therefore, the structure of given amino acid is,

Figure 1
The protection of carboxyl group takes place on reaction with
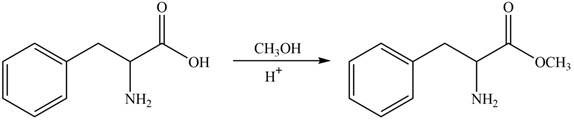
Figure 2
The products formed by the treatment of given amino acid with
(b)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 27P
The products formed by the treatment of given amino acid with
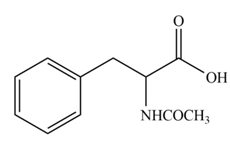
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
The amine group of amino acid form amide bond on reaction with acid chloride as shown in Figure 3.
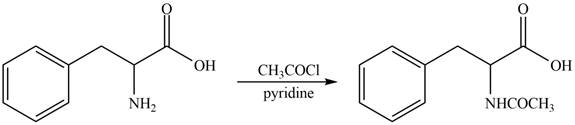
Figure 3
The products formed by the treatment of given amino acid with
(c)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 27P
The products formed by the treatment of given amino acid with
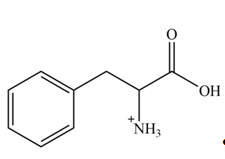
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
The amine group of amino acid form amide bond on reaction with acid chloride as shown in Figure 4.
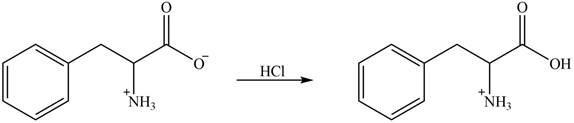
Figure 4
The products formed by the treatment of given amino acid with
(d)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 27P
The products formed by the treatment of given amino acid with
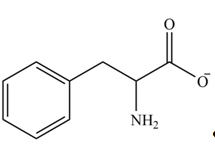
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
The amine group of amino acid form amide bond on reaction with acid chloride as shown in Figure 5.
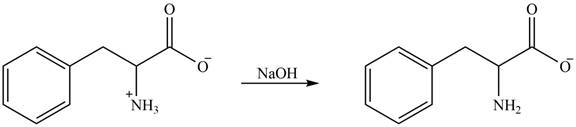
Figure 5
The products formed by the treatment of given amino acid with
(e)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 27P
The products formed by the treatment of given amino acid with
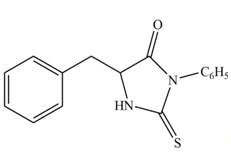
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
The terminal amine group of amino acid on reaction with phenyl isothiocyanate form N-phenylthiohydantoin as shown in Figure 6.
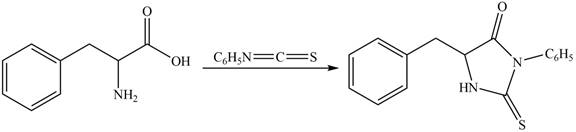
Figure 6
The products formed by the treatment of given amino acid with
Want to see more full solutions like this?
Chapter 27 Solutions
ORGANIC CHEMISTRY BOOK& SG/SM
- State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forward
- Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forwardhelp me solve this hwarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





