
Concept explainers
(a)
Interpretation: The product formed when the given compound in Problem 27.6 undergoes photochemical electrocyclic ring opening or ring closure is to be predicted. The given process is to be labeled as conrotatory or disrotatory. The stereochemistry around tetrahedral stereogenic centers and double bonds is to be indicated.
Concept introduction: Electrocyclic reactions involve the conversion of
Curved arrows aid in determining the movement and flow of electrons in the reaction. The electrons that take part in the
Answer to Problem 27.8P
The product formed in the given photochemical electrocyclic ring closure reaction is
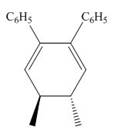
Explanation of Solution
The thermal electrocyclic ring closure reaction is shown below.
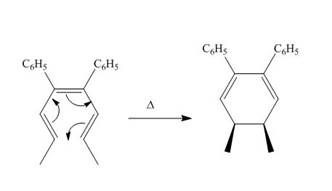
Figure 1
Electrocyclic reactions involve the conversion of
Under photochemical condition, conrotatory process takes place in the given reaction. The orientation of substituents and stereochemistry around tetrahedral stereogenic centers and double bonds in the given reaction are shown below.
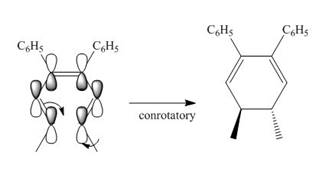
Figure 2
The conrotatory process involves the rotation of two orbitals in the same direction. The reaction in above figure implies that orbitals move in the same direction in the reactant molecule and the formation of trans product occurs. One methyl group is present above the plane and one methyl group is present below the plane in the product. Therefore, the product formed in the given photochemical electrocyclic ring closure reaction is
The product formed in the given photochemical electrocyclic ring closure reaction is
(b)
Interpretation: The product formed when the given compound in Problem 27.6 undergoes photochemical electrocyclic ring opening or ring closure is to be predicted. The given process is to be labeled as conrotatory or disrotatory. The stereochemistry around tetrahedral stereogenic centers and double bonds is to be indicated.
Concept introduction: Electrocyclic reactions involve the conversion of
Curved arrows aid in determining the movement and flow of electrons in the reaction. The electrons that take part in the chemical reactions are shown by the curved arrows.
Answer to Problem 27.8P
The product formed in the given photochemical electrocyclic ring opening reaction is
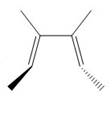
Explanation of Solution
The thermal electrocyclic ring opening reaction is shown below.
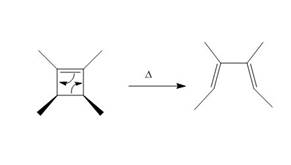
Figure 3
Electrocyclic reactions involve the conversion of
Under photochemical condition, disrotatory process takes place in the given reaction. The movement of substituents and stereochemistry around tetrahedral stereogenic centers and double bonds in the given reaction are shown below.
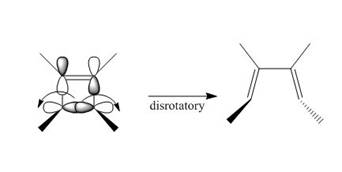
Figure 4
The disrotatory process involves the rotation of two orbitals in the opposite direction. The reaction in above figure implies that orbitals move in the opposite direction in the reactant molecule and the formation of trans product occurs. One methyl group is present above the plane and one methyl group is present below the plane in the product shows the stereochemistry. Therefore, the product formed in the given photochemical electrocyclic ring opening reaction is
The product formed in the given photochemical electrocyclic ring opening reaction is
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
- -Page: 8 nsition metal ions have high-spin aqua complexes except one: [Co(HO)₁]". What is the d-configuration, oxidation state of the metal in [Co(H:O))"? Name and draw the geometry of [Co(H2O)]? b) Draw energy diagrams showing the splitting of the five d orbitals of Co for the two possible electron configurations of [Co(H2O)]: Knowing that A = 16 750 cm and Пl. = 21 000 cm, calculate the configuration energy (.e., balance or ligand-field stabilization energy and pairing energy) for both low spin and high spin configurations of [Co(H2O)]. Which configuration seems more stable at this point of the analysis? (Note that 349.76 cm = 1 kJ/mol) Exchange energy (IT) was not taken into account in part (d), but it plays a role. Assuming exchange an occur within t29 and within eg (but not between tz, and ea), how many exchanges are possible in the low in configuration vs in the high spin configuration? What can you say about the importance of exchange energy 07arrow_forwardDraw everything please on a piece of paper explaining each steparrow_forwardDefine crystalline, polycrystalline and amorphous materials What crystal system and Bravais lattices are shown in the figure immediately below? What do a, b, C, a, ẞ and y represent and what are their values? You can label the Bravais lattices directly above or under the figure. C aarrow_forward
- 32. The diagrams below show the band structure of an intrinsic semiconductor at absolute zero and room temperature. Room Temperature EF E OK Ep- a) In the space below, sketch a similar pair of diagrams for an n-type semiconductor. D) Give the definition and an example of (i) an intrinsic semiconductor and (ii) an n-type semiconductor.arrow_forward29. a) i Which energy diagram best represents the d-electrons in tetrahedral [Co(NH3)4]²+? b) ii c) iii d) iv 11 ་ ↑↓ ↑t t ↑↓ ↑↓ e) none of these ii In1 According to Slater's rules, what is the effective nuclear charge experienced by a 3d electron in 30. Ge? a) 32.00 b) 21.15 c) 16.05 d) 14.00 e) 10.85arrow_forwardRegarding Lowis structuros and geometrios, Draw Lewis structures for the following: SOF4, SO, ICI, XeO2F4, SeF and XeO3. For each one, indicate the observed molecular geometry it adopts.arrow_forward
- Explain the following statements with equations: - The fusion product of an organic compund with sodium metal is an alkaline solution - The test for elements should be done before the solubility tests. - Using less sodium than the organic compound in the ignition test might cause a problem to detect the presence of the nitrogen and sulfur - Formation of colored product when adding ferric acid chloride to phenol solutionarrow_forward31 Indicate the symbol, mass number and the atomic number of the missing product in each of the following nuclear reactions. a) 13/3 N 41 b) 11 Ca 20 c) 90 38 Sr → 133 C + ? + - 6 0 e →? 90 Y + ? 39 11 d) 22 Na → ? + + 1 B +1 β Toarrow_forwardPlease drawarrow_forward
- 9. compore the Following two Venctions IN termy Of Ronction Rate and explan in detail the reasoning that led to your conclusion +He p₁₂ 11- ㅐ 15 .. +He H #H H / H b. Compare the Following too reactions 14 terms of reaction Rate and explain in detail the reasoning that led to your conclusion Н d-C- tłu Na +2446 е -ll +2n "Harrow_forwarda. •Write all of the possible products For the Following ronction А ----- H - H H + H₂0 H+ Н b. in Rite the complete reaction Mechaniszn For the Formation of each product. ·C. Suggest what Reaction conditions could Result in each product being the major Product of the veaction:arrow_forwarda. Write the product For each of the Following reactions H 6-836-6 레 +H₂ N A H A-C-C=C-C-CH + 2 Na +2 NH3 - H H b. Write the reaction Mechanism For. reaction eacharrow_forward
