
Concept explainers
(a)
Interpretation:
The explanation for alanine which has different optical rotation in water, 1 M HCl, and 1 MNaOH is to be stated.
Concept introduction:
The amino acid is made of two
Answer to Problem 27.61AP
Each solvent rotates the alanine molecule to a different angle due to the formation of different complex formation and so it gives rise to different optical rotation in different solvents.
Explanation of Solution
The structure of alanine is shown below.
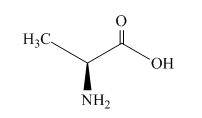
Figure 1
Alanine is an optically active compound. It rotates the plane of polarized light. The reaction of alanine with water, 1 M HCl, and 1 MNaOH forms a complex which is shown below.
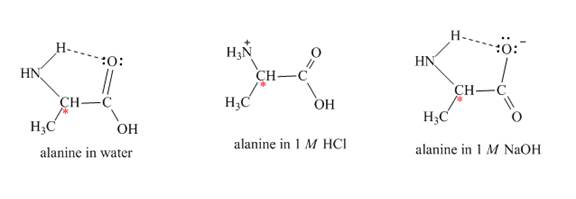
Figure 2
The optical rotation of alanine is measure by Circular Dichroism (CD). It involves circularly polarized light absorption. It measures the angle at which the plane-polarized light is rotated by the molecule. Each solvent rotates the alanine molecule to a different angle, due to this it gives rise to different optical rotation in different solvents.
The alanine has different optical rotation in water, 1 M HCl, and 1 MNaOH. This is because of different solvent molecule rotates the alanine molecule to a different angle.
(b)
Interpretation:
The explanation for two known mono −N− acetyl derivatives of lysine is to be stated.
Concept introduction:
The amino acid is made of two functional groups an amine group, −NH2 and a carboxyl group, −COOH. Along with a side chain is also attached to the center carbon atom due to which each amino acid is distinguished. The amino acid lysine contains a −(CH2)4NH2 side chain.
Answer to Problem 27.61AP
Due to the presence of two amine groups in lysine molecule. It may undergo acetylation reaction from either side and form mono −N− acetyl derivatives of lysine.
Explanation of Solution
The structure of amino acid lysine is shown below.
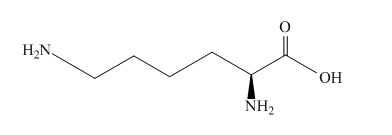
Figure 3
The structure of the lysine molecule contains two amine group one at the α carbon and other at the side chain. Therefore, it has the potential to undergo acetylation reaction from two sides which results in the formation of two mono −N− acetyl derivatives of lysine. The product obtained after the acetylation reaction of lysine is shown below.
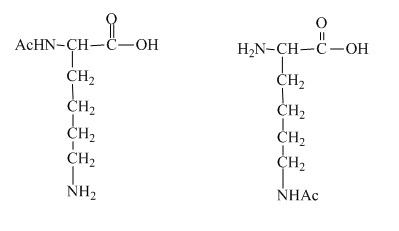
Figure 4
The two mono −N− acetyl derivatives of lysine are possible. This is because of the presence of two amine groups which undergo acetylation reaction from either side and form mono −N− acetyl derivatives of lysine.
(c)
Interpretation:
The explanation for fact that the peptide Gly−Ala−Arg−Ala−Glu which is hydrolyzed by trypsin in water at pH=8 but remains inert to trypsin in 8 M urea pH=8 is to be stated.
Concept introduction:
The amino acid is made of two functional groups an amine group −NH2 and a carboxyl group −COOH. Along with a side chain is also attached to the center carbon atom due to which each amino acid is distinguished. Urea at basic conditions is known to be a denaturation agent of protein.
Answer to Problem 27.61AP
The compound urea under basic conditions acts as a denaturation agent which breakdown the protein molecule bonding. Due to this, the peptide, Gly−Ala−Arg−Ala−Glu which is hydrolyzed by trypsin in the water at pH=8 but remains inert to trypsin in 8 M urea pH=8.
Explanation of Solution
The protein molecule is composed of four types of structure primary, secondary, tertiary and quarternary. The enzyme trypsin hydrolyzes the peptide, Gly−Ala−Arg−Ala−Glu in the water at pH=8 which results in the dissociation of an amide linkage. When the same peptide is treated with urea at same pH, it breaks the tertiary and quarternary structure of the protein and does not hydrolyze the peptide. It causes denaturation of protein by breaking the internal hydrogen bonding of the protein molecule.
The peptide Gly−Ala−Arg−Ala−Glu which is hydrolyzed by trypsin in the water at pH=8 but remains inert to trypsin in 8 M urea pH=8. This is because of urea under basic conditions acts as a denaturation agent which breakdown the protein molecule bonding.
(d)
Interpretation:
The explanation for the peptide containing cysteine on reaction with HSCH2CH2OH and aziridine may cleaved by trypsin at the modified cysteine residue is to be stated.
Concept introduction:
The amino acid is made of two functional groups an amine group, −NH2 and a carboxyl group, −COOH. Along with a side chain is also attached to the center carbon atom due to which each amino acid is distinguished. The amino acid cysteine contains a −CH2SH side chain.
Answer to Problem 27.61AP
The generation of lysine type molecule at the end of the reaction which is cleaved by trypsin enzyme. Due to this, the peptide containing cysteine on reaction with HSCH2CH2OH and aziridine may cleaved by trypsin at the modified cysteine residue.
Explanation of Solution
The structure of the cysteine molecule is shown below.
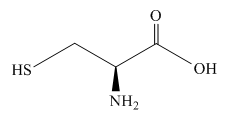
Figure 5
The same molecule in peptides exists as a disulfide bond. The disulfide bond of cysteine in the protein molecule is shown below.
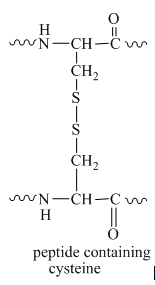
Figure 6
When this protein molecule with a disulfide bond is treated with HSCH2CH2OH which dissociates the disulfide bond. It results in the formation of a thiol group and 2,2'−disulfanediyldiethanol. The reaction of a disulfide bond with HSCH2CH2OH is shown below.
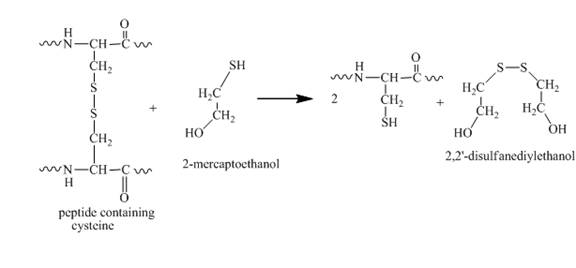
Figure 7
The thiol group is then reacted with the aziridine molecule which results in the formation of a lysine type molecule. This reaction is shown below.
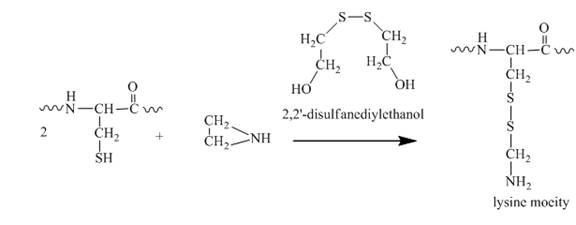
Figure 8
This lysine type molecule then reacts with trypsin enzyme which cleaves arginine and lysine molecule. Due to this, the trypsin enzyme reacts with the modified cysteine residues.
The peptide containing cysteine on reaction with HSCH2CH2OH and aziridine may cleaved by trypsin at the modified cysteine residue. This is because of the generation of lysine type molecule at the end of the reaction which is cleaved by trypsin enzyme.
(e)
Interpretation:
The explanation for the formation of two separable methionine sulfoxides from the oxidation of L−methionine with H2O2 is to be stated.
Concept introduction:
The amino acid is made of two functional groups an amine group, −NH2 and a carboxyl group, −COOH. Along with a side chain is also attached to the center carbon atom due to which each amino acid is distinguished. The amino acid methionine contains −CH2CH2SCH3 side chain.
Answer to Problem 27.61AP
The application of a certain amount of energy which converts one form to another form of structure. Due to this, the formation of two separable methionine sulfoxides from the oxidation of L−methionine with H2O2 is possible.
Explanation of Solution
The structure of methionine is shown below.
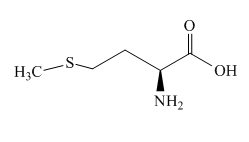
Figure 9
The resonance structure of sulfoxides with two different groups is shown below.
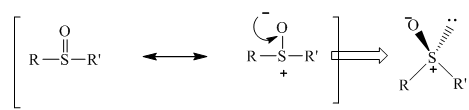
Figure 10
The conversion of one structure to another structure at room temperature requires a certain amount of energy. Therefore, on the application of that amount of energy, the two forms of L−methionine which are separable at room temperature are formed. The structure of two different forms of L−methionine is shown below.
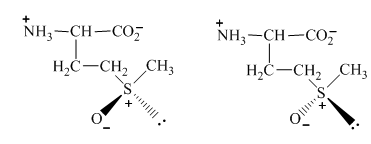
Figure 11
The formation of two separable methionine sulfoxides from the oxidation of L−methionine with H2O2 is possible. This is because of the application of a certain amount of energy which converts one form to another form of structure. They are separable at room temperature.
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- In GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forward
- Given a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardComplete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forward
- Please help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward
- 4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



