
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
8th Edition
ISBN: 9781337687539
Author: Brown/Iverson/Anslyn/ Foote
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 27.21P
Both norepinephrine and epinephrine are synthesized from the same protein-derived amino acid. From which amino acid are they synthesized? What types of reactions are involved in their biosynthesis?
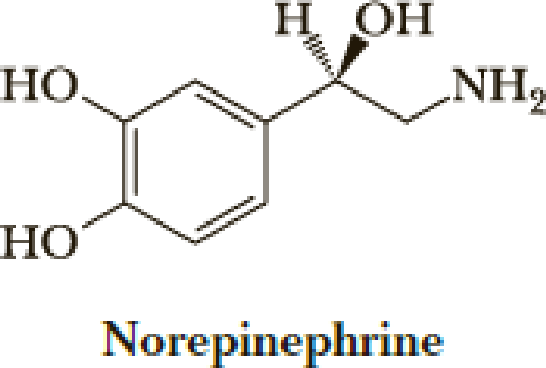
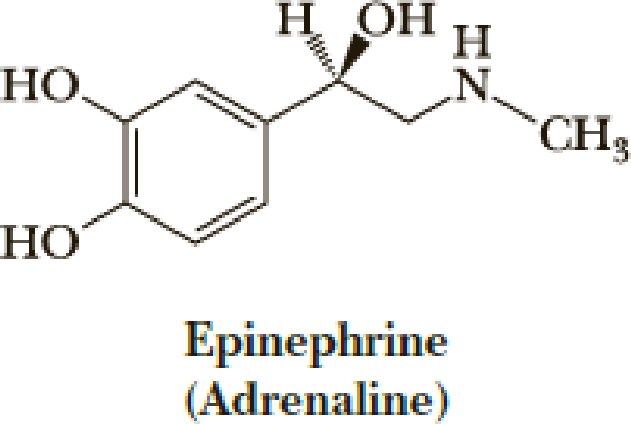
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Chapter 27 Solutions
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
Ch. 27.1 - Of the 20 protein-derived amino acids shown in...Ch. 27.2 - Prob. 27.2PCh. 27.2 - Prob. 27.3PCh. 27.3 - Draw a structural formula for Lys-Phe-Ala. Label...Ch. 27.4 - Which of these tripeptides are hydrolyzed by...Ch. 27.4 - Deduce the amino acid sequence of an undecapeptide...Ch. 27.6 - Prob. 27.7PCh. 27 - What amino acid does each abbreviation stand for?...Ch. 27 - The configuration of the chiral center in -amino...Ch. 27 - Assign an R or S configuration to the chiral...
Ch. 27 - Prob. 27.11PCh. 27 - Prob. 27.12PCh. 27 - Draw zwitterion forms of these amino acids. (a)...Ch. 27 - Prob. 27.14PCh. 27 - Why is Arg often referred to as a basic amino...Ch. 27 - Prob. 27.16PCh. 27 - Prob. 27.17PCh. 27 - Prob. 27.18PCh. 27 - Prob. 27.19PCh. 27 - Prob. 27.20PCh. 27 - Both norepinephrine and epinephrine are...Ch. 27 - Prob. 27.22PCh. 27 - Draw a structural formula for the form of each...Ch. 27 - Prob. 27.24PCh. 27 - Write the zwitterion form of alanine and show its...Ch. 27 - Prob. 27.26PCh. 27 - Write the form of aspartic acid most prevalent at...Ch. 27 - Prob. 27.28PCh. 27 - Prob. 27.29PCh. 27 - For lysine and arginine, the isoelectric point,...Ch. 27 - Prob. 27.31PCh. 27 - Account for the fact that the isoelectric point of...Ch. 27 - Prob. 27.33PCh. 27 - Prob. 27.34PCh. 27 - At pH 7.4, the pH of blood plasma, do the majority...Ch. 27 - Prob. 27.36PCh. 27 - Prob. 27.37PCh. 27 - Prob. 27.38PCh. 27 - A chemically modified guanidino group is present...Ch. 27 - Draw a structural formula for the product formed...Ch. 27 - Prob. 27.41PCh. 27 - Prob. 27.42PCh. 27 - A decapeptide has the following amino acid...Ch. 27 - Following is the primary structure of glucagon, a...Ch. 27 - Prob. 27.45PCh. 27 - Draw a structural formula of these tripeptides....Ch. 27 - Estimate the pI of each tripeptide in Problem...Ch. 27 - Glutathione (G-SH), one of the most common...Ch. 27 - Following are a structural formula and a...Ch. 27 - Prob. 27.50PCh. 27 - Prob. 27.51PCh. 27 - Prob. 27.52PCh. 27 - Prob. 27.53PCh. 27 - Prob. 27.54PCh. 27 - Distinguish between intermolecular and...Ch. 27 - Prob. 27.56PCh. 27 - Prob. 27.57P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY