
Organic Chemistry, Third Edition Binder Ready Version
3rd Edition
ISBN: 9781119110453
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 26.4, Problem 12PTS
Interpretation Introduction
Interpretation:
Mechanism for transesterification of tristearin using methanol in presence of acid catalyst need to be drawn.
Concept introduction:
Transesterification is a process in which the alcohol group present in the carboxylate of triglyceride is exchanged by another organic alcohol group. The general scheme can be shown as,
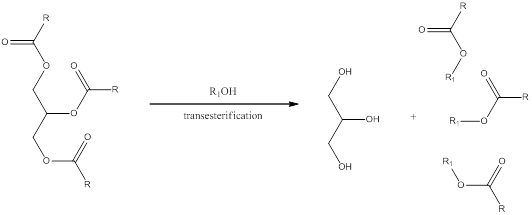
The steps involved in transesterification is,
- Protononation
- Nucleophilic attack
- Deprotonation
- Protonation
- Loss of leaving group
- Deprotonation
To draw: the mechanism of transesterification of tristearin using methanol in presence of acid catalyst.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction.
it is spontaneous only at High T, it is spontaneous at low T
it is nonspontaneous at all T
it is spontanrous at all T.
it is non spontaneous only at low T.
The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction
Which of the following has the largest standard molar entropy, S° (298.15 K)
He
H2
NaCl
KBr
Hg
Chapter 26 Solutions
Organic Chemistry, Third Edition Binder Ready Version
Ch. 26.2 - Prob. 1CCCh. 26.2 - Prob. 2CCCh. 26.3 - Prob. 1LTSCh. 26.3 - Prob. 3PTSCh. 26.3 - Prob. 4ATSCh. 26.3 - Prob. 5ATSCh. 26.3 - Prob. 6ATSCh. 26.4 - Prob. 7CCCh. 26.4 - Prob. 8CCCh. 26.4 - Prob. 2LTS
Ch. 26.4 - Prob. 9PTSCh. 26.4 - Prob. 10ATSCh. 26.4 - Prob. 11ATSCh. 26.4 - Prob. 3LTSCh. 26.4 - Prob. 12PTSCh. 26.4 - Prob. 13LTSCh. 26.4 - Prob. 14LTSCh. 26.5 - Prob. 15CCCh. 26.5 - Prob. 16CCCh. 26.5 - Prob. 17CCCh. 26.5 - Prob. 18CCCh. 26.5 - Prob. 19CCCh. 26.6 - Prob. 20CCCh. 26.6 - Prob. 21CCCh. 26.6 - Prob. 22CCCh. 26.6 - Prob. 23CCCh. 26.7 - Prob. 24CCCh. 26.8 - Prob. 4LTSCh. 26.8 - Prob. 25PTSCh. 26.8 - Prob. 26ATSCh. 26.8 - Prob. 27CCCh. 26.8 - Prob. 28CCCh. 26 - Prob. 29PPCh. 26 - Prob. 30PPCh. 26 - Draw two different cephalins that contain one...Ch. 26 - Prob. 32PPCh. 26 - Prob. 33PPCh. 26 - Prob. 34PPCh. 26 - Prob. 35PPCh. 26 - Prob. 36PPCh. 26 - Prob. 37PPCh. 26 - Prob. 38PPCh. 26 - Prob. 39PPCh. 26 - Prob. 40PPCh. 26 - Prob. 41PPCh. 26 - Prob. 42PPCh. 26 - Prob. 43PPCh. 26 - Prob. 44PPCh. 26 - Prob. 45PPCh. 26 - Prob. 46PPCh. 26 - Prob. 47PPCh. 26 - Prob. 48PPCh. 26 - Prob. 49PPCh. 26 - Prob. 50PPCh. 26 - Prob. 51IPCh. 26 - Prob. 52IPCh. 26 - Prob. 53IPCh. 26 - Prob. 54IPCh. 26 - Prob. 55IPCh. 26 - Prob. 56CPCh. 26 - Prob. 57CP
Knowledge Booster
Similar questions
- Which of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forwardWhat is the major product of the following reaction? O O OH OH 1. BH 2. H₂O₂, NaOH OH OHarrow_forwardDraw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forward
- Draw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forwardWhat is the unsaturation number for compounds with the formula C₂H₁₂Cl₂? O õ õ o o 4 3arrow_forwardIndicate the product obtained (formula). F3C. CF3 Br NH2 NH OMe K2CO3, DABCO, DMFarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY