
ORG CHEM CONNECT CARD
6th Edition
ISBN: 9781264860746
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26.2, Problem 3P
Label each stereogenic center as R or S.
a. 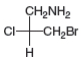 b.
b. 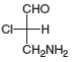 c.
c. 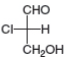 d.
d. 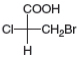
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
b.
CH3
H3C
'N'
H3C
CH3
CN
Ph
1.
OH
N
2. H2O2, Pyridine
For each of the Followin, moleaks draw all OF
The Resonance contributing stuluctures and
compare these three molecules in terms of
Resonance stabilization
1-C-1
a.
b.
H
A-C+
О
112-1
C.
F-C-F
F
a. Explain Why electron withdrawing groupe
tend to be meta-Directors. Your answer Should
lyclude all apropriate. Resonance contributing
Structures
6.
Explain why -ll is an ortho -pura
drccton evon though chlorine has a very High
Electronegativity
Chapter 26 Solutions
ORG CHEM CONNECT CARD
Ch. 26.2 - Prob. 1PCh. 26.2 - Prob. 2PCh. 26.2 - Label each stereogenic center as R or S. a. b. c....Ch. 26.2 - Convert the ball-and-stick model to a Fischer...Ch. 26.2 - Prob. 5PCh. 26.2 - Prob. 6PCh. 26.3 - Prob. 7PCh. 26.3 - Prob. 8PCh. 26.4 - Prob. 9PCh. 26.4 - Prob. 10P
Ch. 26.6 - Prob. 11PCh. 26.6 - Prob. 12PCh. 26.6 - Prob. 13PCh. 26.6 - Prob. 14PCh. 26.6 - Prob. 15PCh. 26.7 - Prob. 16PCh. 26.7 - Draw a stepwise mechanism for the following...Ch. 26.7 - Prob. 18PCh. 26.8 - Prob. 19PCh. 26.9 - Prob. 20PCh. 26.9 - Prob. 21PCh. 26.9 - Draw the products formed when D-arabinose is...Ch. 26.9 - Prob. 23PCh. 26.10 - Prob. 24PCh. 26.10 - Prob. 25PCh. 26.10 - Prob. 26PCh. 26.10 - Prob. 27PCh. 26.11 - Prob. 28PCh. 26.11 - Prob. 29PCh. 26.12 - Prob. 30PCh. 26.12 - Prob. 31PCh. 26.13 - Prob. 32PCh. 26.13 - Prob. 33PCh. 26.13 - Problem-28.35
Draw the structures of the...Ch. 26.13 - Prob. 35PCh. 26 - 28.37 Convert each ball-and-stick model to a...Ch. 26 - Prob. 37PCh. 26 - Prob. 38PCh. 26 - 28.40 Convert each compound to a Fischer...Ch. 26 - Prob. 40PCh. 26 - Prob. 41PCh. 26 - 28.43 Draw a Haworth projection for each compound...Ch. 26 - Prob. 43PCh. 26 - 28.45 Draw both pyranose anomers of each...Ch. 26 - Prob. 45PCh. 26 - 28.50 Draw the products formed when D-altrose is...Ch. 26 - 28.58 Draw a stepwise mechanism for the following...Ch. 26 - Prob. 62PCh. 26 - Prob. 63PCh. 26 - Prob. 64PCh. 26 - Prob. 65PCh. 26 - Prob. 66PCh. 26 - Prob. 67PCh. 26 - Prob. 68PCh. 26 - Prob. 69PCh. 26 - Prob. 70P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forwardIndicate what the Luther equation is used for?arrow_forward
- Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forward
- Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License