
(a)
Interpretation:
The R or S configuration to each of the chiral C atom in the given structure is to be assigned.
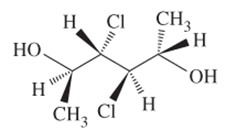
Concept introduction:
Enantiomers are optical isomers which can rotate the plane polarized light either clockwise or anticlockwise. These molecules must have at least one chiral C atom which is bonded with four different groups. They are assigned by R and S configuration. The R/S naming follows the Cahn-Ingold-Prelog Rules for naming the enantiomer as R or S-enantiomer. According to Cahn-Ingold-Prelog Rules assign the numbers from 1 to 4 to the groups bonded with chiral C atom on the basis of their molar mass. The essential condition is that the 4th group or atom must be below the plane means with dash line. If 1(2(3 is clockwise it will be R-configuration and if it is anticlockwise it will be S-configuration.
If the 4th number group is in plane then follow these steps.
- Swap the 4 group with the group with dash line
- Assign R/S configuration with clockwise or anticlockwise movement
- Flip the configuration to get the real configuration.
If the 4th number group is in above the plane shown by wedge line then follow these steps.
- Swap the 4 group with the group with dash line.
- Assign R/S configuration with clockwise or anticlockwise movement.
(b)
Interpretation:
The R or S configuration to each of the chiral C atom in the given structure should be assigned.
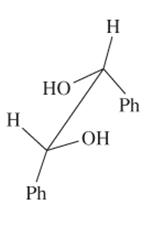
Concept introduction:
Enantiomers are optical isomers which can rotate the plane polarized light either clockwise or anticlockwise. These molecules must have at least one chiral C atom which is bonded with four different groups. They are assigned by R and S configuration. The R/S naming follows the Cahn-Ingold-Prelog Rules for naming the enantiomer as R or S-enantiomer. According to Cahn-Ingold-Prelog Rules assign the numbers from 1 to 4 to the groups bonded with chiral C atom on the basis of their molar mass. The essential condition is that the 4th group or atom must be below the plane means with dash line. If 1(2(3 is clockwise it will be R-configuration and if it is anticlockwise it will be S-configuration.
If the 4th number group is in plane then follow these steps.
- Swap the 4 group with the group with dash line
- Assign R/S configuration with clockwise or anticlockwise movement
- Flip the configuration to get the real configuration.
If the 4th number group is in above the plane shown by wedge line then follow these steps.
- Swap the 4 group with the group with dash line.
- Assign R/S configuration with clockwise or anticlockwise movement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
GENERAL CHEMISTRY(LL)-W/MASTERINGCHEM.
- help 20arrow_forwardProvide the drawing of the unknown structure that corresponds with this data.arrow_forward20.44 The Diels-Alder reaction is not limited to making six-membered rings with only car- bon atoms. Predict the products of the following reactions that produce rings with atoms other than carbon in them. OCCH OCCH H (b) CH C(CH₂)s COOCH མ་ནས་བ (c) N=C H -0.X- (e) H C=N COOCHS + CH2=CHCH₂ →→arrow_forward
- 3) Draw a detailed mechanism and predict the product of the reaction shown? 1) EtMgBr 2) H3O+arrow_forwardHow to draw the mechanism for this reaction?arrow_forward> H₂C=C-CH2-CH3 B. H₂O Pt C. + H2 + H₂O H D. 16. Give the IUPAC name for each of the following: B. Cl Cl c. Cl Cl 17. Draw the line-angle formula for each of the following compounds: 1. phenol 2. 1,3-dichlorobenzene 3. 4-ethyltoluene < Previous Submit Assignment Next ▸arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





