
(a)
Interpretation:
Isoprene units in the given set of compounds need to be circled.
Concept introduction:
Terpenes are naturally occurring compounds which share a common feature. All terpenes are thought to be assembled from isoprene units having five carbon atoms. So the structure of terpenes has the carbon atoms in multiples of five only.
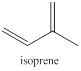
The isoprene units are identified by following few steps namely,
- Identify the number of isoprene units
- Look for methyl group
- Trial and error to find isoprene unit
To identify: the isoprene units in bisabolene
(b)
Interpretation:
Isoprene units in the given set of compounds need to be circled.
Concept introduction:
Terpenes are naturally occurring compounds which share a common feature. All terpenes are thought to be assembled from isoprene units having five carbon atoms. So the structure of terpenes has the carbon atoms in multiples of five only.

The isoprene units are identified by following few steps namely,
- Identify the number of isoprene units
- Look for methyl group
- Trial and error to find isoprene unit
To identify: the isoprene units in flexibilene
(c)
Interpretation:
Isoprene units in the given set of compounds need to be circled.
Concept introduction:
Terpenes are naturally occurring compounds which share a common feature. All terpenes are thought to be assembled from isoprene units having five carbon atoms. So the structure of terpenes has the carbon atoms in multiples of five only.

The isoprene units are identified by following few steps namely,
- Identify the number of isoprene units
- Look for methyl group
- Trial and error to find isoprene unit
To identify: the isoprene units in humulene
(d)
Interpretation:
Isoprene units in the given set of compounds need to be circled.
Concept introduction:
Terpenes are naturally occurring compounds which share a common feature. All terpenes are thought to be assembled from isoprene units having five carbon atoms. So the structure of terpenes has the carbon atoms in multiples of five only.

The isoprene units are identified by following few steps namely,
- Identify the number of isoprene units
- Look for methyl group
- Trial and error to find isoprene unit
To identify: the isoprene units in vitamin A
(e)
Interpretation:
Isoprene units in the given set of compounds need to be circled.
Concept introduction:
Terpenes are naturally occurring compounds which share a common feature. All terpenes are thought to be assembled from isoprene units having five carbon atoms. So the structure of terpenes has the carbon atoms in multiples of five only.

The isoprene units are identified by following few steps namely,
- Identify the number of isoprene units
- Look for methyl group
- Trial and error to find isoprene unit
To identify: the isoprene units in geraniol
(f)
Interpretation:
Isoprene units in the given set of compounds need to be circled.
Concept introduction:
Terpenes are naturally occurring compounds which share a common feature. All terpenes are thought to be assembled from isoprene units having five carbon atoms. So the structure of terpenes has the carbon atoms in multiples of five only.

The isoprene units are identified by following few steps namely,
- Identify the number of isoprene units
- Look for methyl group
- Trial and error to find isoprene unit
To identify: the isoprene units in sabinene
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL)-W/WILEYPLUS
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





