
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26, Problem 44P
Draw both pyranose anomers of each aldohexose using a three-dimensional representation with a chair pyranose. Label each anomer as
a. 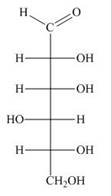 b.
b. 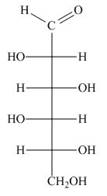
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the reaction mechanism to predict the product of the transformation below:
N
H
?
H₂O
Provide steps and explanation.
Steps and explanation.
Chapter 26 Solutions
EBK ORGANIC CHEMISTRY
Ch. 26.2 - Prob. 1PCh. 26.2 - Prob. 2PCh. 26.2 - Label each stereogenic center as R or S. a. b. c....Ch. 26.2 - Convert the ball-and-stick model to a Fischer...Ch. 26.2 - Prob. 5PCh. 26.2 - Prob. 6PCh. 26.3 - Prob. 7PCh. 26.3 - Prob. 8PCh. 26.4 - Prob. 9PCh. 26.4 - Prob. 10P
Ch. 26.6 - Prob. 11PCh. 26.6 - Prob. 12PCh. 26.6 - Prob. 13PCh. 26.6 - Prob. 14PCh. 26.6 - Prob. 15PCh. 26.7 - Prob. 16PCh. 26.7 - Draw a stepwise mechanism for the following...Ch. 26.7 - Prob. 18PCh. 26.8 - Prob. 19PCh. 26.9 - Prob. 20PCh. 26.9 - Prob. 21PCh. 26.9 - Draw the products formed when D-arabinose is...Ch. 26.9 - Prob. 23PCh. 26.10 - Prob. 24PCh. 26.10 - Prob. 25PCh. 26.10 - Prob. 26PCh. 26.10 - Prob. 27PCh. 26.11 - Prob. 28PCh. 26.11 - Prob. 29PCh. 26.12 - Prob. 30PCh. 26.12 - Prob. 31PCh. 26.13 - Prob. 32PCh. 26.13 - Prob. 33PCh. 26.13 - Problem-28.35
Draw the structures of the...Ch. 26.13 - Prob. 35PCh. 26 - 28.37 Convert each ball-and-stick model to a...Ch. 26 - Prob. 37PCh. 26 - Prob. 38PCh. 26 - 28.40 Convert each compound to a Fischer...Ch. 26 - Prob. 40PCh. 26 - Prob. 41PCh. 26 - 28.43 Draw a Haworth projection for each compound...Ch. 26 - Prob. 43PCh. 26 - 28.45 Draw both pyranose anomers of each...Ch. 26 - Prob. 45PCh. 26 - 28.50 Draw the products formed when D-altrose is...Ch. 26 - 28.58 Draw a stepwise mechanism for the following...Ch. 26 - Prob. 62PCh. 26 - Prob. 63PCh. 26 - Prob. 64PCh. 26 - Prob. 65PCh. 26 - Prob. 66PCh. 26 - Prob. 67PCh. 26 - Prob. 68PCh. 26 - Prob. 69PCh. 26 - Prob. 70P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forward
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forward
- Can you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forwardPart 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY