
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Question
Chapter 26, Problem 42PP
Interpretation Introduction
Interpretation:
Compound structure has to be drawn when alcohol with 30 carbon atoms and
Concept introduction:
Hydrolysis is a process in which the ester is converted to its respective carboxylic acid and alcohol in presence of aqueous acid or base.
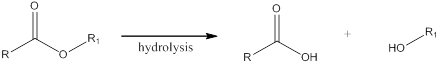
Esterification is a reaction when an alcohol and a carboxylic acid react together in presence of aqueous acid or base to form an ester. The general scheme can be shown as,
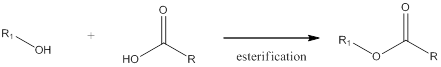
Esterification is the reverse of hydrolysis
To draw: the compound taken when the given carboxylic acid and alcohol are obtained when treated with aqueous sodium hydroxide.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Assign ALL signals for the proton and carbon NMR spectra on the following pages.
7.5
1.93
2.05
C
B
A
4
3
5
The Joh.
9
7
8
1
2
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0 ppm
9
7
8
0.86
OH 10
4
3
5
1
2
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
ppm
9
7
8
CI
4
3
5
1
2
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
2.21
4.00
1.5
2.00
2.07
1.0
ppm
2.76
Assign the functional group bands on the IR spectra.
Chapter 26 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 26.2 - Prob. 1CCCh. 26.2 - Prob. 2CCCh. 26.3 - Prob. 1LTSCh. 26.3 - Prob. 3PTSCh. 26.4 - Prob. 7CCCh. 26.4 - Prob. 8CCCh. 26.4 - Prob. 2LTSCh. 26.4 - Prob. 9PTSCh. 26.4 - Prob. 3LTSCh. 26.4 - Prob. 12PTS
Ch. 26.5 - Prob. 15CCCh. 26.5 - Prob. 16CCCh. 26.5 - Prob. 17CCCh. 26.5 - Prob. 18CCCh. 26.5 - Prob. 19CCCh. 26.6 - Prob. 20CCCh. 26.6 - Prob. 21CCCh. 26.6 - Prob. 22CCCh. 26.6 - Prob. 23CCCh. 26.7 - Prob. 24CCCh. 26.8 - Prob. 4LTSCh. 26.8 - Prob. 25PTSCh. 26.8 - Prob. 27CCCh. 26.8 - Prob. 28CCCh. 26 - Prob. 29PPCh. 26 - Prob. 30PPCh. 26 - Prob. 32PPCh. 26 - Prob. 33PPCh. 26 - Prob. 34PPCh. 26 - Prob. 35PPCh. 26 - Prob. 36PPCh. 26 - Prob. 37PPCh. 26 - Prob. 38PPCh. 26 - Prob. 39PPCh. 26 - Prob. 40PPCh. 26 - Prob. 41PPCh. 26 - Prob. 42PPCh. 26 - Prob. 43PPCh. 26 - Prob. 44PPCh. 26 - Prob. 45PPCh. 26 - Prob. 46PPCh. 26 - Prob. 47PPCh. 26 - Prob. 48PPCh. 26 - Prob. 49PPCh. 26 - Prob. 50PP
Knowledge Booster
Similar questions
- Find the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forward
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
- Ordene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY