
Concept explainers
Interpretation:
All resonance structures for the product of head-to-tail addition shown in Figure 26-11 are to be drawn. Then curved-arrow notation is to be used to show the movement of electrons in the head-to-tail addition of a growing chain of poly(ethyl acrylate) to a molecule of ethyl acrylate. The resonance structures are to be drawn to explain why head-to tail addition occurs instead of head-to-head addition.
Concept introduction:
Styrene is an unsymmetric vinyl monomer, so we can differentiate between the two carbons of the vinyl group. The less substituted carbon is called the tail, and the more substituted carbon is called the head. As a result, regiochemistry comes into play during
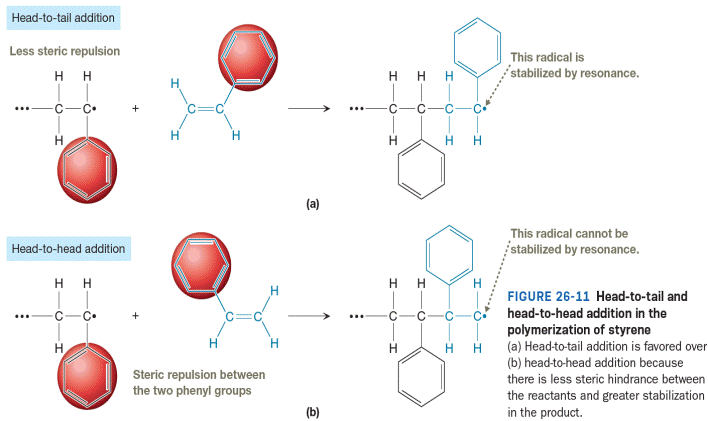
The head-to-tail addition is favored in the polymerization of polystyrene for the two reasons shown in Figure 26-11. First, steric repulsion between the two phenyl groups (one at the end of the propagating radical and one in the monomer) makes it less likely that a head-to-head collision will bring the bond-forming carbons within a bond length. Secondly, the radical formed in the head-to-tail addition is more stable. The radical produced from head-to-tail addition is resonance stabilized while the radical produced from head-to-head addition is not.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Drawingarrow_forwardSo, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forward
- Draw a mental model for calcium chloride mixed with sodium phosphatearrow_forwardhere is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forward
- Drawing of 3-fluro-2methylphenolarrow_forwardWhich compound(s) will be fully deprotonated (>99%) by reaction with one molar equivalent of sodium hydroxide? I, II, III I, || I, III I only II, III SH | H3C-C=C-H || III NH2arrow_forwardWill NBS (and heat or light) work for this reaction, or do we have to use Br2?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
