
Concept explainers
(a)
Interpretation:
The principal organic product that is obtained when
Concept introduction:
The reaction in which an atom of
Answer to Problem 26.26AP
The principal organic product that is obtained when
Explanation of Solution
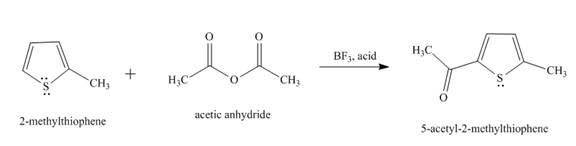
The reaction of
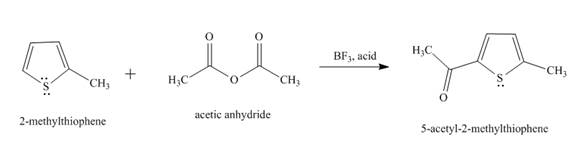
Figure 1
In the above reaction, the second position of
Therefore, the product formed by the above reaction is
The principal organic product,
(b)
Interpretation:
The principal organic product that is obtained when
Concept introduction:
The reaction in which an atom of aromatic system is replaced by an electrophile is known as electrophilic aromatic substitution reaction. The compound, acetic anhydride behaves as a acetylating compound in the presence of
Answer to Problem 26.26AP
The principal organic product that is obtained when
Explanation of Solution
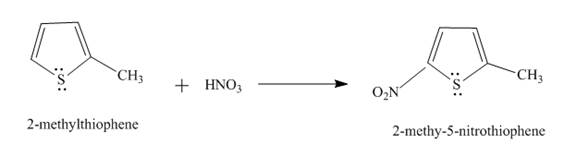
The reaction of
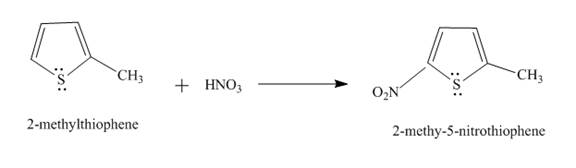
Figure 2
In the above reaction, the second position of
Therefore, the product formed by the above reaction is
The principal organic product,
(c)
Interpretation:
The incomplete reaction between diene and dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
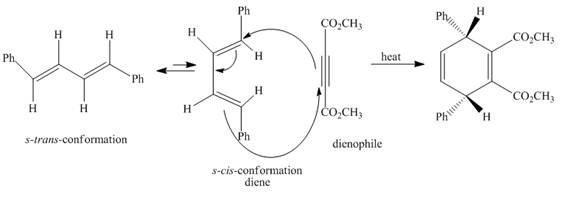
Explanation of Solution
The given incomplete reaction is shown below.
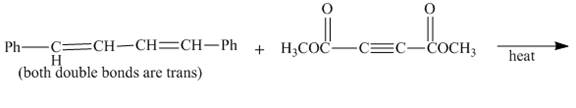
Figure 3
In the above incomplete reaction, the given
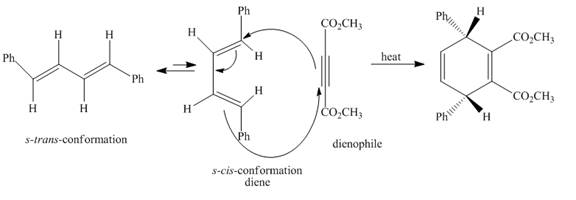
Figure 4
Therefore, one product is obtained from the above shown Diels Alder reaction.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 4.
(d)
Interpretation:
The incomplete reaction between an alkene and benzoquinone is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
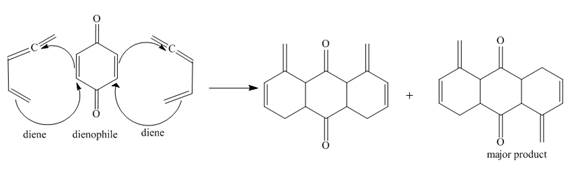
Explanation of Solution
The given incomplete reaction is shown below.
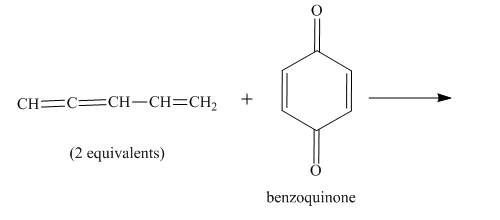
Figure 5
In the above incomplete reaction, the given two equivalents of alkene behaves as a diene and undergoes Diels Alder reaction with benzoquinone which behaves as a dienophile in the presence of heat to form two products as shown below.

Figure 6
Therefore, two products are obtained from the above shown Diels Alder reaction. The second product is the major one because of the less van der Waals repulsion present in between two double bonds which are exocyclic.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 6.
(e)
Interpretation:
The incomplete reaction between a diene and a dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
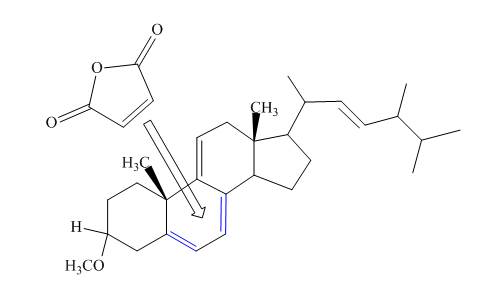
Explanation of Solution
The given incomplete reaction is shown below.
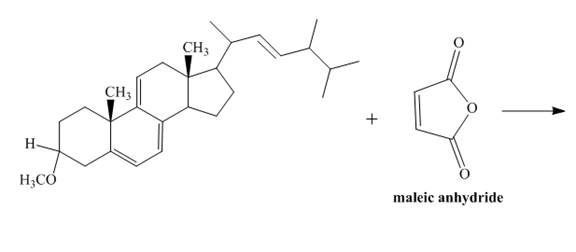
Figure 7
Only the

Figure 8
The above reaction does not form any product because the
Therefore, no product is formed in the above shown reaction.
There is no formation of the product takes place in the given reaction.
(f)
Interpretation:
The incomplete reaction between a diene and a dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
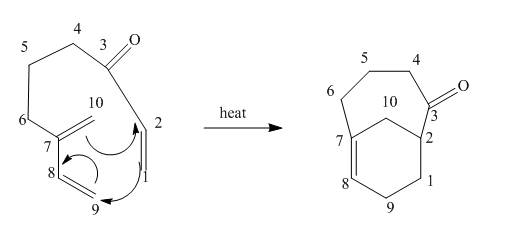
Explanation of Solution
The given incomplete reaction is shown below.
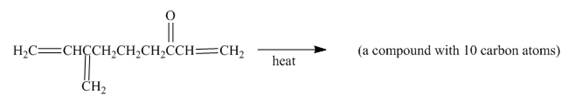
Figure 9
In the above incomplete reaction, the intramolecular Diels Alder reaction takes place in the presence of heat to form two products as shown below.

Figure 10
In the above reaction, diene and dienophile are present in the same compound. Therefore, two products are obtained from the above shown Diels Alder reaction. Therefore, the shifting of bonds takes place within the molecule to form a single product.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 10.
(g)
Interpretation:
The incomplete reaction between nickel choride and
Concept introduction:
Metallocene compounds are composed of an electropositive metal ions specially
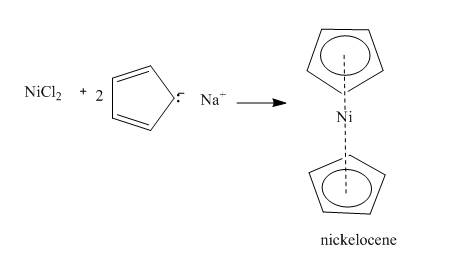
Answer to Problem 26.26AP
The complete reaction is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
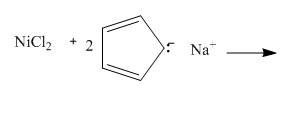
Figure 11
In the above incomplete reaction, the nickel chloride reacts with

Figure 12
Therefore, the reaction between nickel chloride and
The complete reaction corresponding to the incomplete reaction between nickel choride and
Want to see more full solutions like this?
Chapter 26 Solutions
EBK ORGANIC CHEMISTRY
- H-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forwardDraw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

