
Combine the following structures to create a ribonucleotide. Show where water is removed to form an N-glycosidic linkage and where water is removed to form a phosphate ester. Draw the resulting ribonucleotide structure, and name it.
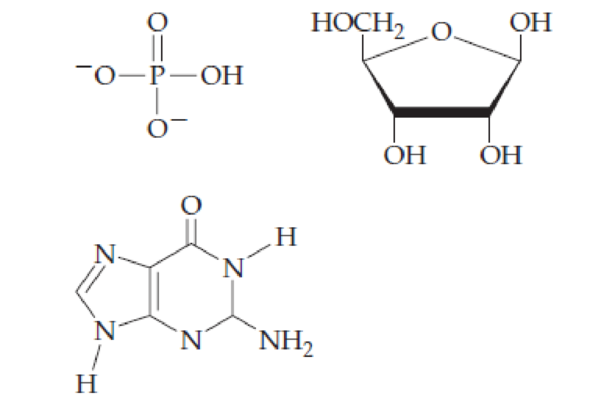
Interpretation:
The ribonucleotide structure has to be predicted and named.
Concept Introduction:
Composition of nucleic acid: Nucleic acid is a polymer of nucleotides. Each nucleotide has three parts: a sugar, a nitrogenous base, and a phosphate group.
Sugar: In both DNA and RNA, sugar portion is found. In DNA, the sugar is D-ribose, where at 2’hydroxyl group is absent and in RNA, the hydroxyl group is present at 2’.
Nitrogenous bases: Five types of nitrogenous bases (has unique one-letter code A, G, T, U, and C) are derived from two parent compounds called purine and pyrimidine. The purine derivatives are Adenine and Guanine are two fused rings. The pyrimidine derivatives are six-membered nitrogen containing ring. Adenine, Guanine, Thymine, and Cytosine are the nitrogenous bases present in DNA. Adenine, Guanine, Cytosine and Uracil are the nitrogenous bases present in RNA.
Nucleotide: (Nucleoside + phosphate)
Nucleotides are the building blocks of nuclei acids; monomers of DNA and RNA polymers. At carbon-5’ of the ribose sugar, a phosphate group is added which is collectively known as nucleotide. Phosphate groups can be added to any of the nucleotide to form diphosphate or triphosphate.
Nucleoside and its naming: The combination of monosaccharide (sugar) and nitrogenous base is known as nucleoside. The nucleoside names are the nitrogenous base name modified with criteria. While naming nucleoside of purine derivatives the suffix ‘-osine’ is included and for pyrimidine derivatives the suffix ‘-idine’ is used. No prefix used for the nucleosides containing ribose and the prefix ‘deoxy-’ is used for deoxyribose.
Naming nucleotide: At the end of the nucleoside, phosphate group is added. For example, 5’-monophosphate means adding one phosphate group at 5’carbon in the sugar ring.
Numbering the atoms in sugar and base rings:
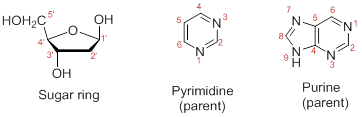
In order to distinguish the atoms in the sugar of a nucleoside and atoms of a base ring, numbers without prime is used for atoms in the base ring and numbers with prime used for the atoms in the sugar ring.
Answer to Problem 26.22UKC
The structure is,
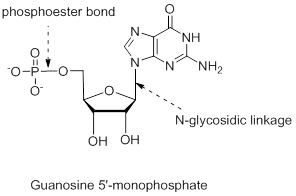
Explanation of Solution
The nucleotide structure is a combination of nucleoside [ribose (presence of –OH group at 2’ carbon atom in the sugar ring) and guanine base (derivative of purine parent)]. The nucleoside name is the nitrogenous name itself; thus, name is Guanine. Looking at the criteria the name of nitrogenous base is modified as follows, the suffix ‘-osine’ is used for the purine derivatives, as here it is Adenine to Guanosine.
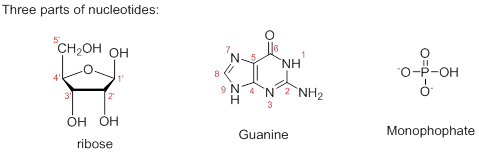
Therefore, the structure of given Guanosine 5’-monophosphate is,
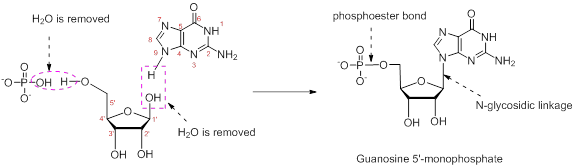
The ribonucleotide structure is predicted and named.
Want to see more full solutions like this?
Chapter 26 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Edition; Modified Mastering Chemistry with Pearson eText -- ValuePack ... and Biological Chemistry (4th Edition)
Additional Science Textbook Solutions
Biological Science (6th Edition)
Biology: Life on Earth (11th Edition)
Genetics: From Genes to Genomes
Fundamentals of Anatomy & Physiology (11th Edition)
Chemistry & Chemical Reactivity
Campbell Essential Biology (7th Edition)
- Biochemistry Please help. Thank you When carbamyl phosphate is joined to L-ornathine, where does the energy for the reaction come from?arrow_forwardBiochemistry Question Please help. Thank you What is the function of glutamate dehydrogenase?arrow_forwardBiochemistry Question Please help. Thank you How and why does a high protein diet affect the enzymes of the urea cycle?arrow_forward
- Biochemistry What is the importance of the glucose-alanine cycle?arrow_forwardBiochemistry Assuming 2.5 molecules of ATP per oxidation of NADH/(H+) and 1.5molecules of ATP per oxidation of FADH2, how many ATP are produced per molecule of pyruvate? Please help. Thank youarrow_forward1. How would you explain the term ‘good food’? 2. How would you define Nutrition? 3. Nutrients are generally categorised into two forms. Discuss.arrow_forward
- Biochemistry Question. Please help solve. Thank you! Based upon knowledge of oxidation of bioorganic compounds and howmuch energy is released during their oxidation, rank the following, from most to least, with respect to how much energy would be produced from each during their oxidation. Explain your placement for each one.arrow_forwardBiochemistry Question.For the metabolism of amino acids what is the first step for theirbreakdown? Why is it necessary for this breakdown product to be transported to the liver? For the catabolism of the carbon backbone of these amino acids, there are 7 entry points into the “standard” metabolic pathways. List these 7 entry points and which amino acids are metabolized to these entry points. Please help. Thank you!arrow_forwardBiochemistry Question. Please help. Thank you. You are studying pyruvate utilization in mammals for ATP production under aerobic conditions and have synthesized pyruvate with Carbon #1 labelled with radioactive C14. After only one complete cycle of the TCA cycle, which of the TCA cycle intermediates would be labeled with C14? Explain your answer. Interestingly, you find C14 being excreted in the urine. How does it get there?arrow_forward
- Biochemistry question. Please help with. Thanks in advance For each of the enzymes listed below, explain what the enzyme does including function, names (or structures) of the substrate and products and the pathway(s) (if applicable) it is/are found in. (a) ATP synthetase (b) succinate dehydrogenase (c) isocitrate lyase (d) acetyl CoA carboxylase (e) isocitrate dehydrogenase (f) malate dehydrogenasearrow_forwardDraw and name each alcohol and classify it as primary, secondary, or tertiary. Explain your answer thoroughly.arrow_forwardDraw the product of each reaction. If there are multiple products, draw only the major product. Explain your answer thoroughly.arrow_forward
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning





