
(a)
Interpretation:
The structure of polyisobutylene is to be drawn.
Concept Introduction:
(a)
Answer to Problem 26.21SP
The structure of polyisobutylene is shown in Figure 1.
Explanation of Solution
Polyisobutylene formed from the cationic polymerization of isobutylene which proceeds through the formation of tertiary cation at the carbon containing two methyl groups. Therefore, the structure of polyisobutylene polymer is,
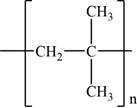
Figure 1: Structure of polyisobutylene.
(b)
Interpretation:
The polymerization of isobutylene is an addition or condensation polymerization is to be stated.
Concept Introduction:
Polymerization is the process of formation of a long chain polymer by the reaction of one or two monomer. The product formed in the polymerization contains the repeated units of the monomer from which it is formed.
(b)
Answer to Problem 26.21SP
The polymerization of isobutylene to form butyl rubber is an addition polymerization.
Explanation of Solution
Addition polymerization is the polymerization in which polymer is formed by the addition of several monomer units to form a long chain polymer. It generally consists of one monomer unit.
Condensation polymerization is the polymerization in which two or more monomers combine to form a larger product along with the loss of water molecules. The resultant product repeatedly combines with another product to form long chain polymer.
The polymerization of isobutylene involves the addition of several monomer units to form a long chain polymer. Therefore, this is an example of addition polymerization.
(c)
Interpretation:
The polymerization of isobutylene would be most appropriate in cationic, anionic or free radical polymerization condition is to be predicted.
Concept Introduction:
Polymerization is the process of formation of a long chain polymer by the reaction of one or two monomer. The product formed in the polymerization contains the repeated units of the monomer from which it is formed.
(c)
Answer to Problem 26.21SP
The polymerization of isobutylene to form butyl rubber proceeds in most appropriate way through cationic polymerization.
Explanation of Solution
The formation of a long chain polymer becomes favorable only due to the stability of intermediate formed in the chain propagating step. Higher is the stability of cation, anion or free radical formed in the polymerization reaction, more favorable will be the polymerization process. In case of isobutylene, one carbon atom contains two electron donating groups which increase electron density on the carbon atom. Therefore, the formation of cation is more favorable in case of isobutylene as it forms tertiary cation and stabilized by two electron donating groups. Thus, the polymerization of isobutylene occurs in the most appropriate way through cationic polymerization.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry (9th Edition)
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





