
Concept explainers
a)
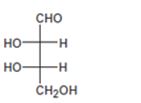
Interpretation:
The configuration as R or S is to be assigned to each chirality center in the monosaccharide given and whether it is a D sugar or L sugar is to be stated.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
In Fischer projections, D sugars have the hydroxyl group on right at the farthest chirality center and L sugars have this hydroxyl group on left.
To assign:
The configuration as R or S to each chirality center in the monosaccharide given and to state whether it is a D sugar or L sugar.
b)
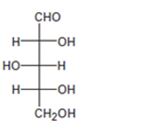
Interpretation:
The configuration as R or S is to be assigned to each chirality center in the monosaccharide given and whether it is a D sugar or L sugar is to be stated.
Concept introduction:
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To assign:
The configuration as R or S to each chirality center in the monosaccharide given and to state whether it is a D sugar or L sugar.
c)
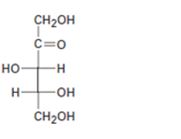
Interpretation:
The configuration as R or S is to be assigned to each chirality center in the monosaccharide given and whether it is a D sugar or L sugar is to be stated.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To assign:
The configuration as R or S to each chirality center in the monosaccharide given and to state whether it is a D sugar or L sugar.
Trending nowThis is a popular solution!

Chapter 25 Solutions
ORGANIC CHEMISTRY W/OWL
- Which of the m/z values corresponds to the base peak in the mass spectrum shown? 100 80 A. 45 B. 44 C. 29 D. 15 Intensity 20 0 10 20 30 40 B- m/z -8 50 E. 30 Which of the m/z values correspond to the molecular ion for the compound shown? A. 18 B. 82 OH C. 100 D. 102 E. 103arrow_forwardCan someone help me with drawing my arrows.arrow_forwardCan I get help drawing my arrows #2arrow_forward
- Can I get some help with my arrows? I have included what the final outcome needs to look like. #3arrow_forwardPlease explain how to calculate the pH.arrow_forwardI'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





