
Concept explainers
(a)
Interpretation: The product formed on thermal electrocyclic ring opening of the given compounds is to be predicted.
Concept introduction: Electrocyclic reactions involve ring opening or ring closure in a conjugated polyene. According to Woodward-Hoffmann rules, the polyene containing even number of bonds in thermal conditions undergoes reaction in conrotatory fashion and polyene containing odd number of bonds undergo reaction in disrotatory fashion.
Answer to Problem 25P
The product formed on thermal electrocyclic ring opening of the compound A is,
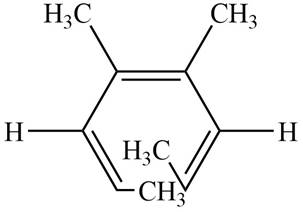
Figure 1
The product formed on thermal electrocyclic ring opening of the compound B is,
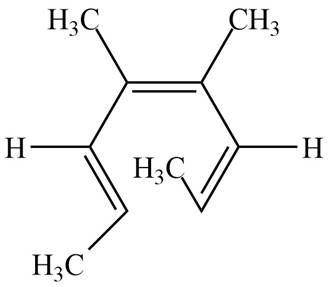
Figure 2
Explanation of Solution
The given compound A is,
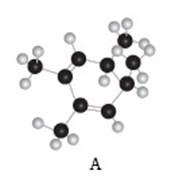
Figure 3
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
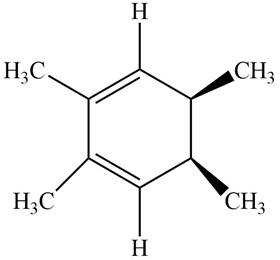
Figure 4
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding
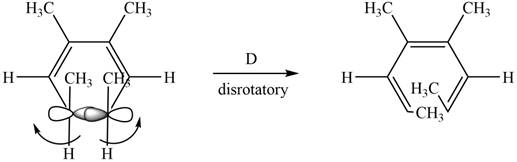
Figure 5
The given compound B is,
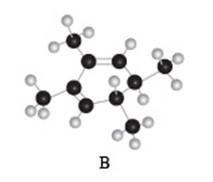
Figure 6
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
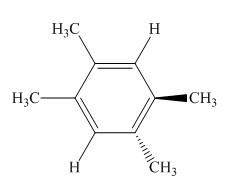
Figure 7
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding chemical reaction is shown below.
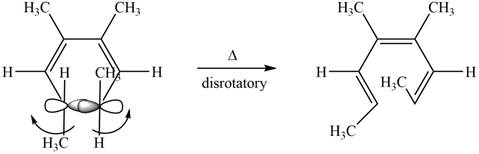
Figure 8
The products formed on thermal electrocyclic ring opening of the compound A and compound B is are shown in Figure 1 and Figure 2.
(b)
Interpretation: The product formed on photochemical electrocyclic ring opening of the given compounds.
Concept introduction: Electrocyclic reactions involve ring opening or ring closure in a conjugated polyene. According to Woodward-Hoffmann rules, the polyene containing even number of bonds in photochemical conditions undergoes reaction in disrotatory fashion and polyene containing odd number of bonds undergo reaction in conrotatory fashion.
Answer to Problem 25P
The product formed on photochemical electrocyclic ring opening of the compound A is,
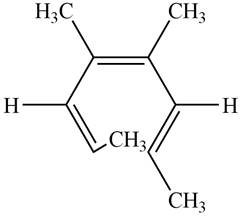
Figure 9
The product formed on photochemical electrocyclic ring opening of the compound B is,
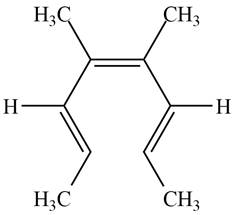
Figure 10
Explanation of Solution
The given compound A is,
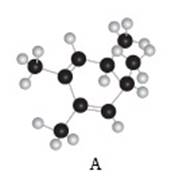
Figure 3
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
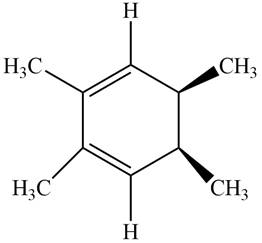
Figure 4
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding chemical reaction is shown below.
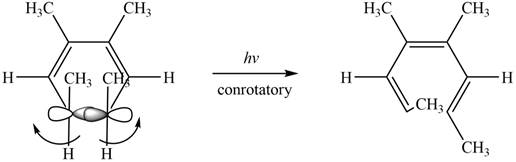
Figure 11
The given compound B is,
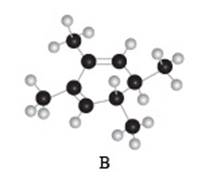
Figure 6
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,

Figure 7
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding chemical reaction is shown below.
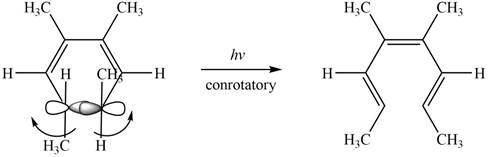
Figure 12
The products formed on photochemical electrocyclic ring opening of the compound A and compound B is are shown in Figure 9 and Figure 10.
Want to see more full solutions like this?
Chapter 25 Solutions
ORG CHEM CONNECT CARD
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





