
Concept explainers
(a)
Interpretation:
The sequence of steps for the given reaction involving the adenylation of aspartic acid, is to be determined.
Concept Introduction:
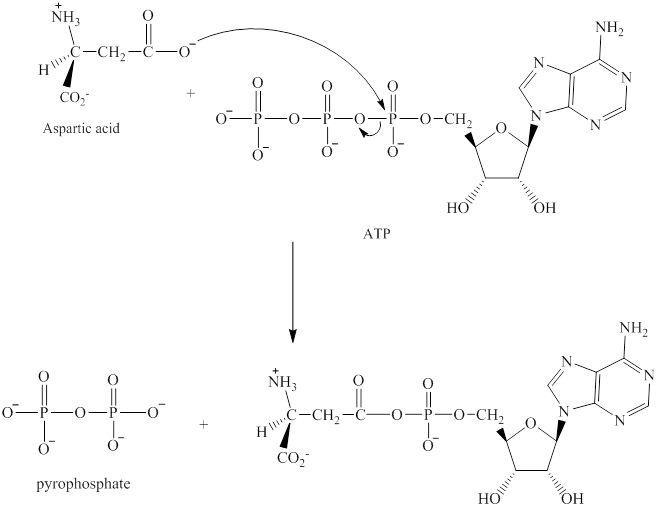
The adenylation of aspartic acid takes place in three steps. In the first step, aspartic acid reacts with ATP. The pyrophosphate is the leaving group in this
Answer to Problem 25.40AP
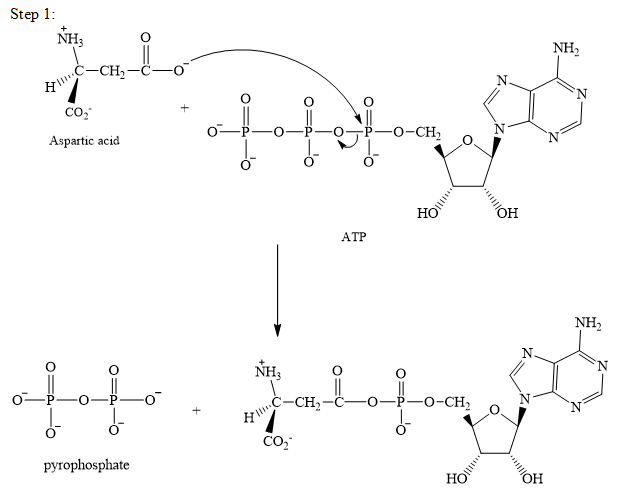
Step 2:
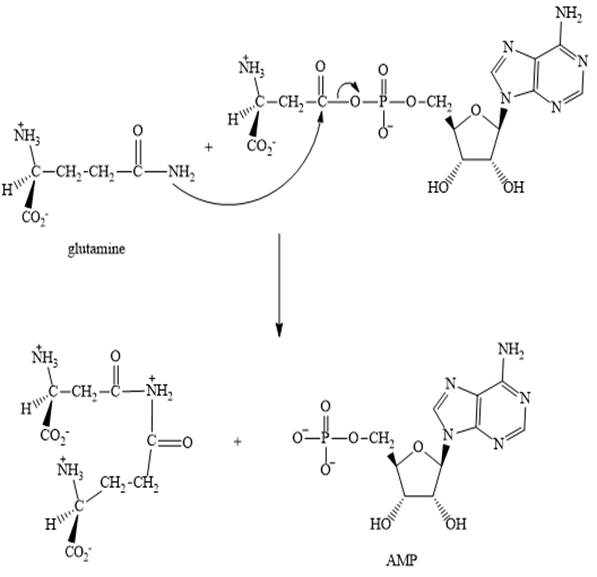
Step 3:
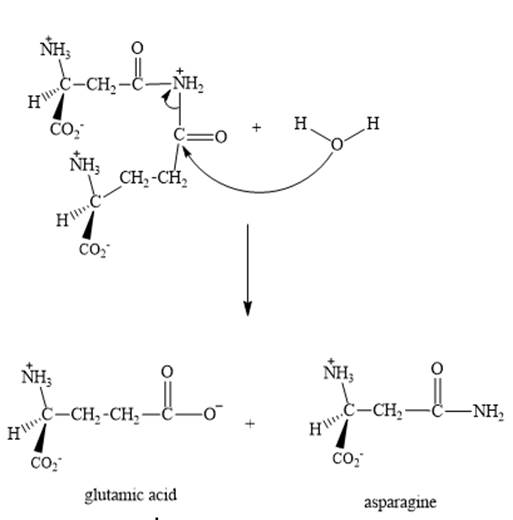
Explanation of Solution
The adenylation of aspartic acid takes place in three steps.
Step 1: In the first step, aspartic acid reacts with ATP. The pyrophosphate is the leaving group in this
Step 2: In the second step, glutamine displaces AMP by a nucleophilic acyl substitution reaction.
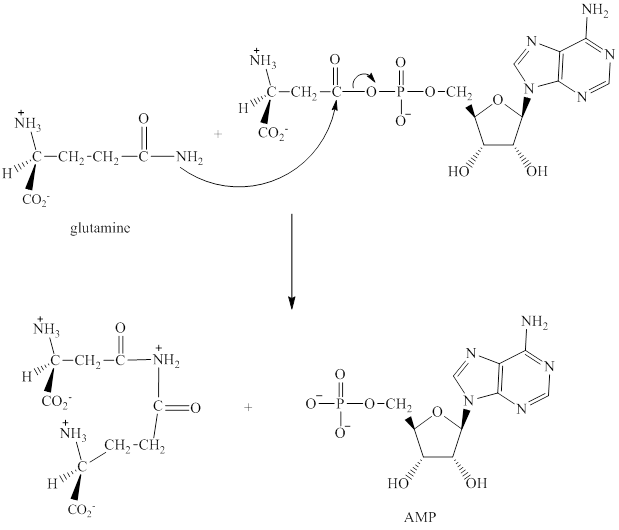
Step 3: The intermediate produced in the step 2 undergoes hydrolysis to the form of glutamic acid and asparagine.
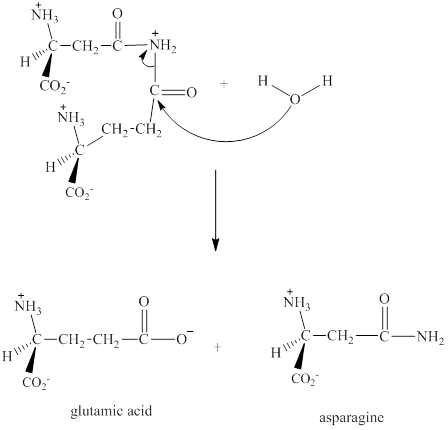
The sequence of steps for the given reaction involving the adenylation of aspartic acid is as shown above.
(b)
Interpretation:
The utilization of ATP in the equilibrium between glutamine and asparagine, is to be explained.
Concept Introduction:
ATP hydrolysis reactions are highly favorable. The reaction is used to help less favorable reactions which occur in high yield. In the adenylation of aspartic acid, the AMP group is a better leaving group than the hydroxyl group.
Answer to Problem 25.40AP
ATP is highly energetically favorable and produces AMP, which is a better leaving group than
Explanation of Solution
To make the less favorable reactions occur in high yield, the high energetic favorability of ATP is utilized. AMP is a better leaving group than
ATP is highly energetically favorable and produces AMP, which is a better leaving group than
(c)
Interpretation:
The
Concept Introduction:
The free energy change of two or more reactions can be used to determine the free energy change of a third reaction. The reactions are treated just like algebraic equations.
Answer to Problem 25.40AP
Explanation of Solution
_____________________________________________________________________
The
(d)
Interpretation:
The meaning of ATP hydrolysis in biosynthesis of asparagine is to be explained.
Concept Introduction:
ATP hydrolysis is a high energy reaction. It drives other reactions coupled to it, to completion.
Answer to Problem 25.40AP
If ATP is removed from the process, then the reaction is unfavorable. ATP drives the reaction to completion.
Explanation of Solution
If ATP is removed from the process, then the reaction is unfavorable. ATP does not actually undergo hydrolysis, but when coupled to the adenylation of aspartic acid, it becomes favorable and drives the reactions towards completion.
If ATP is removed from the process, then the reaction is unfavorable. ATP drives the reaction to completion.
(e)
Interpretation:
The reaction that ensures the completion of asparagine biosynthesis, is to be determined.
Concept Introduction:
Pyrophosphatase catalyzes the irreversible conversion of pyrophosphate to phosphate. This reaction ensures the completion of asparagine biosynthesis.
Answer to Problem 25.40AP
The other reaction which drives asparagine synthesis towards completion is the conversion of pyrophosphate to phosphate.
Explanation of Solution
The other reaction which drives asparagine synthesis towards completion is the conversion of pyrophosphate to phosphate, which is irreversible. Pyrophosphate is generated as a by-product during the adenylation of aspartic acid.
The other reaction which drives asparagine synthesis towards completion is the conversion of pyrophosphate to phosphate, which is irreversible.
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





