
Concept explainers
(a)
Interpretation:
The sequence of steps for the given reaction involving the adenylation of aspartic acid, is to be determined.
Concept Introduction:
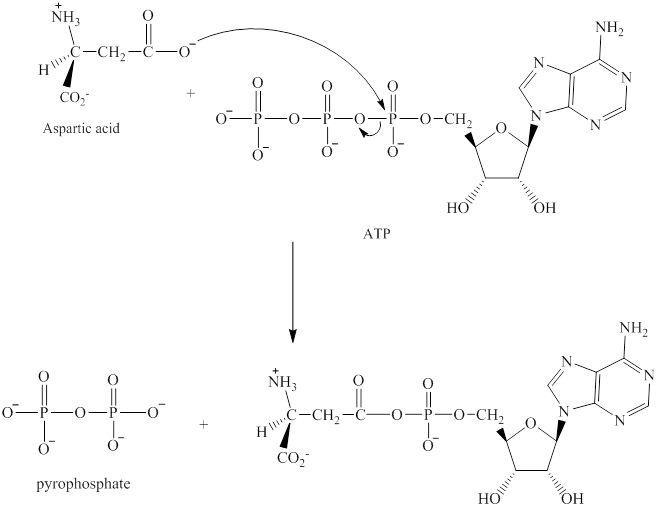
The adenylation of aspartic acid takes place in three steps. In the first step, aspartic acid reacts with ATP. The pyrophosphate is the leaving group in this
Answer to Problem 25.40AP
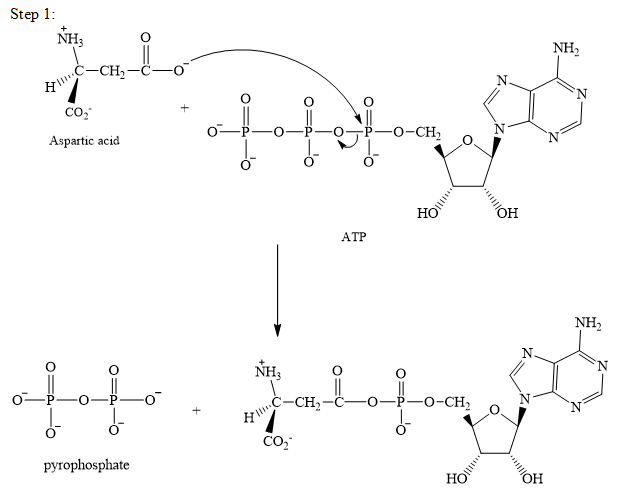
Step 2:
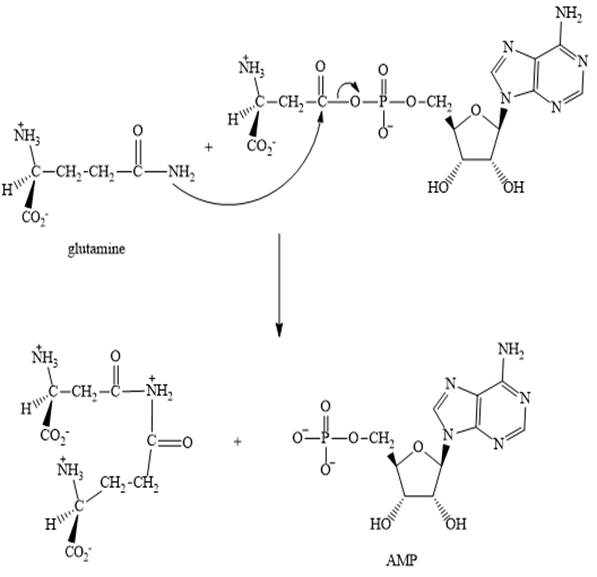
Step 3:
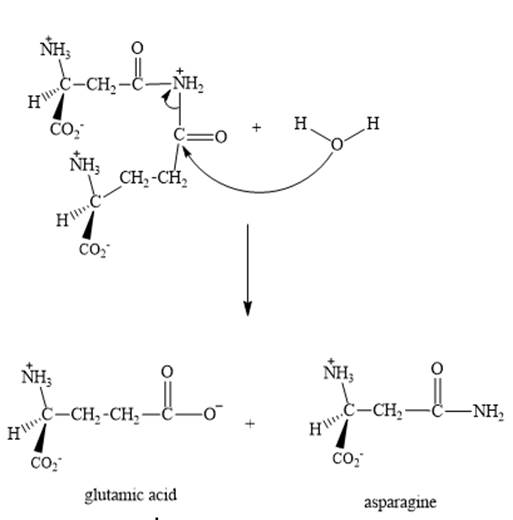
Explanation of Solution
The adenylation of aspartic acid takes place in three steps.
Step 1: In the first step, aspartic acid reacts with ATP. The pyrophosphate is the leaving group in this
Step 2: In the second step, glutamine displaces AMP by a nucleophilic acyl substitution reaction.
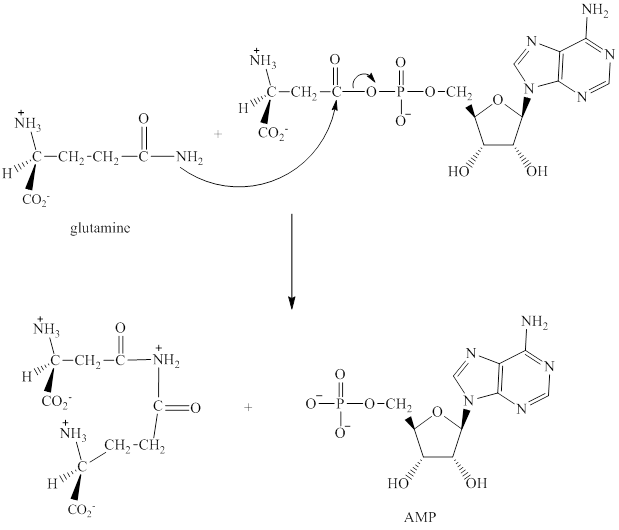
Step 3: The intermediate produced in the step 2 undergoes hydrolysis to the form of glutamic acid and asparagine.
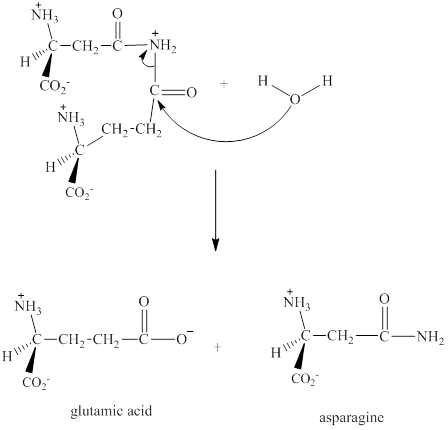
The sequence of steps for the given reaction involving the adenylation of aspartic acid is as shown above.
(b)
Interpretation:
The utilization of ATP in the equilibrium between glutamine and asparagine, is to be explained.
Concept Introduction:
ATP hydrolysis reactions are highly favorable. The reaction is used to help less favorable reactions which occur in high yield. In the adenylation of aspartic acid, the AMP group is a better leaving group than the hydroxyl group.
Answer to Problem 25.40AP
ATP is highly energetically favorable and produces AMP, which is a better leaving group than
Explanation of Solution
To make the less favorable reactions occur in high yield, the high energetic favorability of ATP is utilized. AMP is a better leaving group than
ATP is highly energetically favorable and produces AMP, which is a better leaving group than
(c)
Interpretation:
The
Concept Introduction:
The free energy change of two or more reactions can be used to determine the free energy change of a third reaction. The reactions are treated just like algebraic equations.
Answer to Problem 25.40AP
Explanation of Solution
_____________________________________________________________________
The
(d)
Interpretation:
The meaning of ATP hydrolysis in biosynthesis of asparagine is to be explained.
Concept Introduction:
ATP hydrolysis is a high energy reaction. It drives other reactions coupled to it, to completion.
Answer to Problem 25.40AP
If ATP is removed from the process, then the reaction is unfavorable. ATP drives the reaction to completion.
Explanation of Solution
If ATP is removed from the process, then the reaction is unfavorable. ATP does not actually undergo hydrolysis, but when coupled to the adenylation of aspartic acid, it becomes favorable and drives the reactions towards completion.
If ATP is removed from the process, then the reaction is unfavorable. ATP drives the reaction to completion.
(e)
Interpretation:
The reaction that ensures the completion of asparagine biosynthesis, is to be determined.
Concept Introduction:
Pyrophosphatase catalyzes the irreversible conversion of pyrophosphate to phosphate. This reaction ensures the completion of asparagine biosynthesis.
Answer to Problem 25.40AP
The other reaction which drives asparagine synthesis towards completion is the conversion of pyrophosphate to phosphate.
Explanation of Solution
The other reaction which drives asparagine synthesis towards completion is the conversion of pyrophosphate to phosphate, which is irreversible. Pyrophosphate is generated as a by-product during the adenylation of aspartic acid.
The other reaction which drives asparagine synthesis towards completion is the conversion of pyrophosphate to phosphate, which is irreversible.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry, Ebook And Single-course Homework Access
- Write the systematic name of each organic molecule: structure name × HO OH ☐ OH CI CI O CI OH OHarrow_forwardく Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forward
- theres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forward
- Organic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forward
- Draw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





