
Concept explainers
(a)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(a)
Explanation of Solution
The D-ribose is an aldopentose molecule. The carbonyl group present in D-ribose is
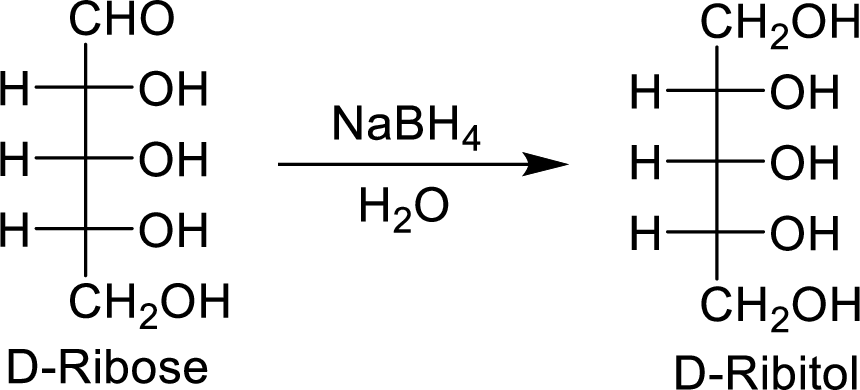
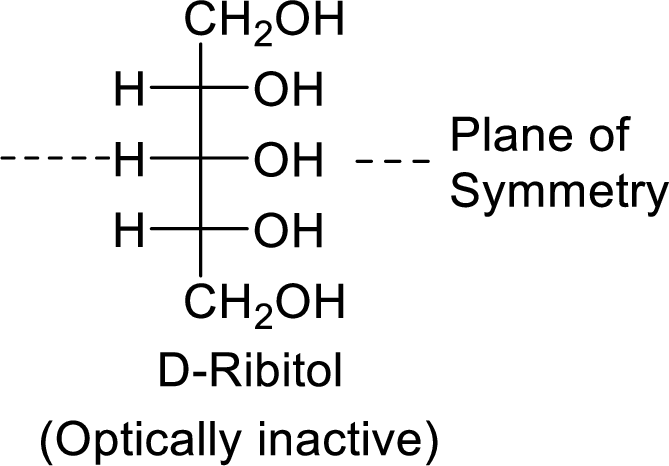
The D-ribitol is a meso compound and shows plane of symmetry. So, it is optically inactive.
(b)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reagent
(b)
Explanation of Solution
When the D-ribose molecule is made to react with
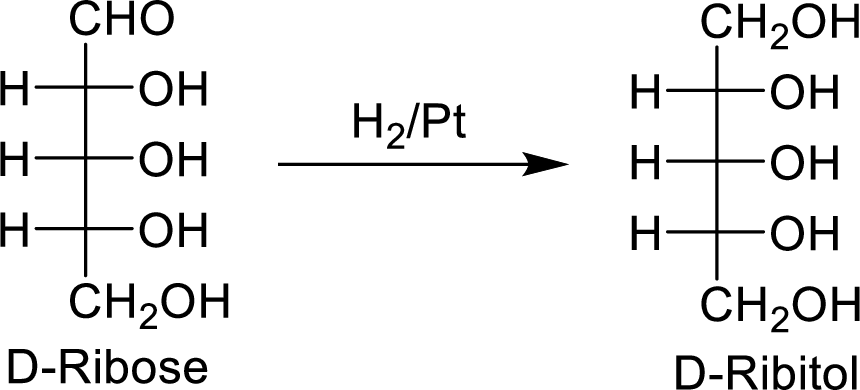
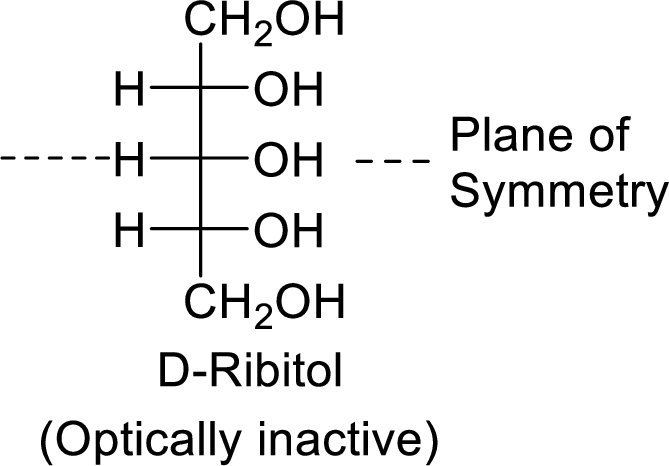
The D-ribitol is a meso compound and shows plane of symmetry. So, it is optically inactive.
(c)
Interpretation:
The product formed by the reaction of D-ribose with warm
Concept Introduction:
The reaction of warm
(c)
Explanation of Solution
The nitric acid (
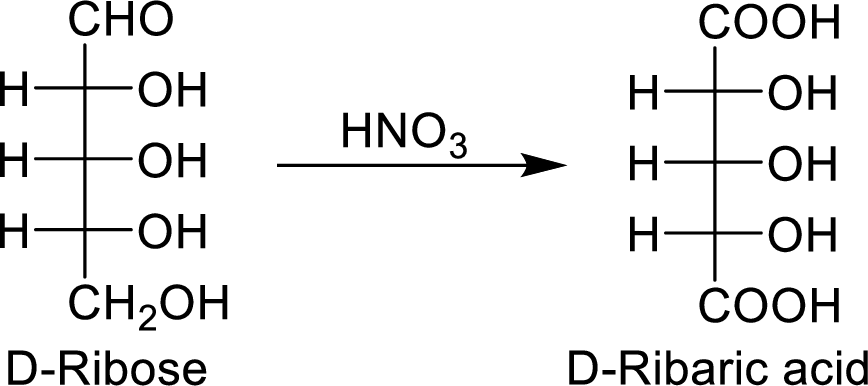
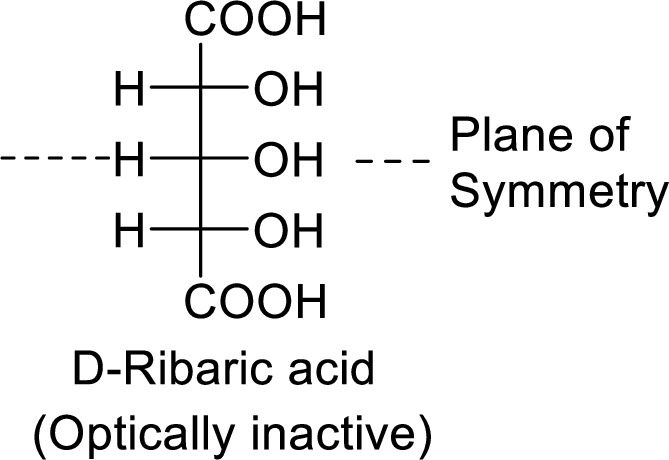
The product formed is a meso compound and shows plane of symmetry. So, it is optically inactive.
(d)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(d)
Explanation of Solution
When the D-ribose reacts with
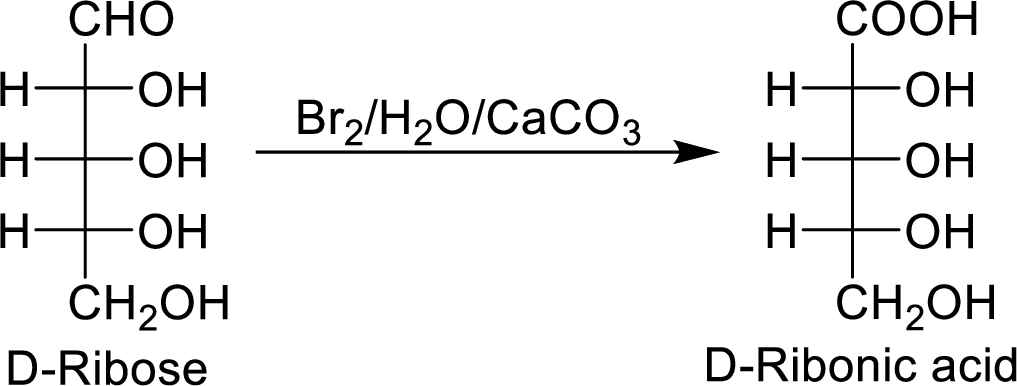
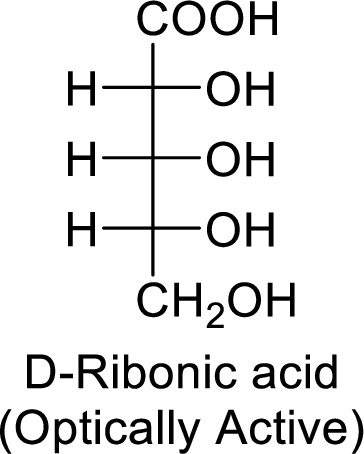
The D-ribonic acid is a chiral molecule and is optically active.
(e)
Interpretation:
The product formed by the reaction of D-ribose with
Concept Introduction:
The reaction of
(e)
Explanation of Solution
The D-ribose molecule consumes
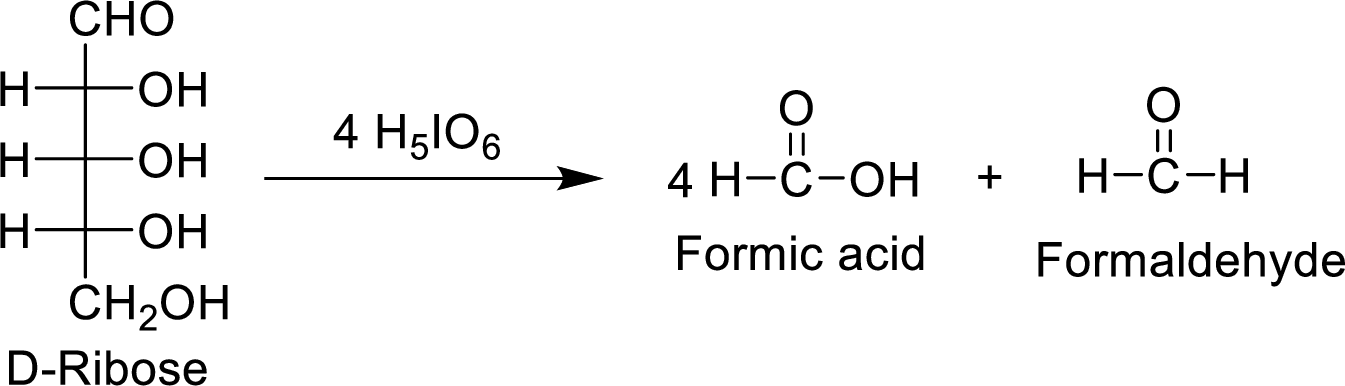
The products formed are achiral and are optically inactive.
(f)
Interpretation:
The product formed by the reaction of D-ribose with aniline (
(f)
Explanation of Solution
The D-galactose molecule reacts with aniline molecule and the aldehyde group of carbohydrate is reacted with the
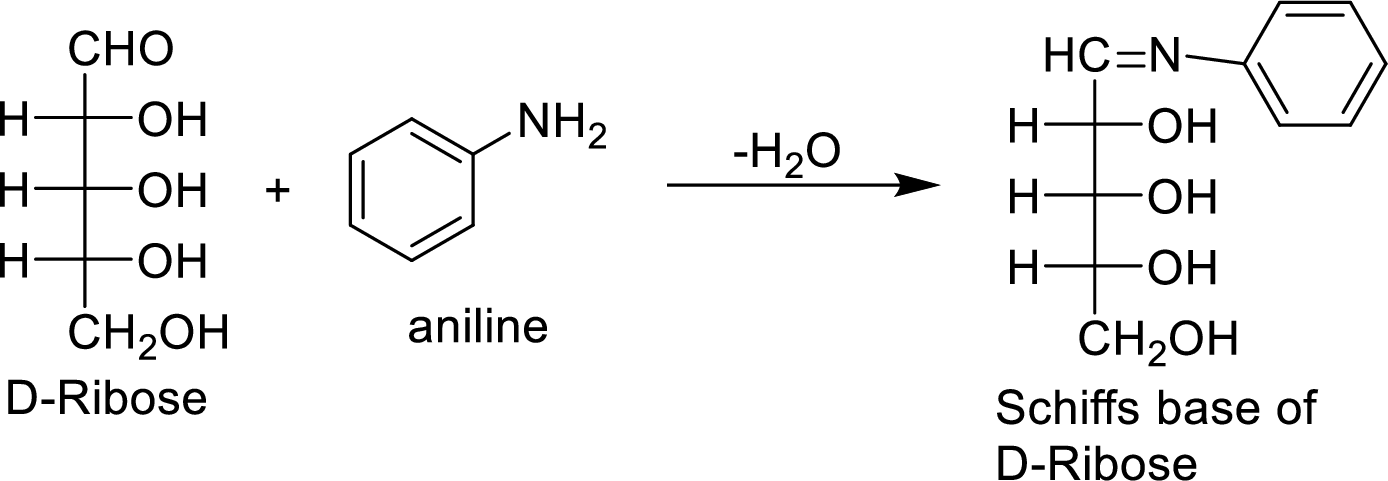
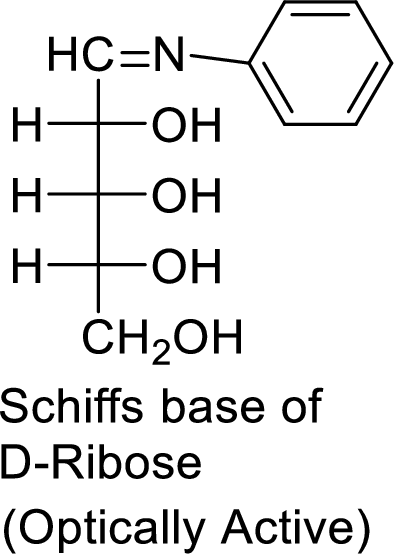
The product formed is chiral and optically active.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- Construct a molecular orbital energy-level diagram for BeH2. Sketch the MO pictures (schematic representation) for the HOMO and LUMO of BeH2 [Orbital Potential Energies, H (1s): -13.6 eV; Be (2s): -9.3 eV, Be (2p): -6.0 eV]arrow_forwardIndicate the isomers of the A(H2O)6Cl3 complex. State the type of isomerism they exhibit and explain it briefly.arrow_forwardState the formula of the compound potassium μ-dihydroxydicobaltate (III) tetraoxalate.arrow_forward
- Consider the reaction of the cyclopentanone derivative shown below. i) NaOCH2CH3 CH3CH2OH, 25°C ii) CH3!arrow_forwardWhat constitutes a 'reference material', and why does its utilization play a critical role in the chemical analysis of food products? Provide examples.arrow_forwardExplain what calibration is and why it is essential in relation to food analysis. Provide examples.arrow_forward
- The cobalt mu-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forwardThe cobalt mi-hydroxide complex cobaltate(III) of potassium is a dinuclear complex. Correct?arrow_forward3. Arrange the different acids in Exercise B # 2 from the strongest (1) to the weakest acid (10). 1. 2. (strongest) 3. 4. 5. 6. 7. 8. 9. 10 10. (weakest)arrow_forward
- Name Section Score Date EXERCISE B pH, pOH, pка, AND PKD CALCULATIONS 1. Complete the following table. Solution [H+] [OH-] PH РОН Nature of Solution A 2 x 10-8 M B 1 x 10-7 M C D 12.3 6.8 2. The following table contains the names, formulas, ka or pka for some common acids. Fill in the blanks in the table. (17 Points) Acid Name Formula Dissociation reaction Ka pka Phosphoric acid H₂PO₁ H3PO4 H++ H₂PO 7.08 x 10-3 Dihydrogen H₂PO H₂PO H+ HPO 6.31 x 10-6 phosphate Hydrogen HPO₁ 12.4 phosphate Carbonic acid H2CO3 Hydrogen HCO 6.35 10.3 carbonate or bicarbonate Acetic acid CH,COOH 4.76 Lactic acid CH₂CHOH- COOH 1.38 x 10 Ammonium NH 5.63 x 10-10 Phenol CH₂OH 1 x 10-10 Protonated form CH3NH3* 3.16 x 10-11 of methylaminearrow_forwardIndicate whether it is true that Co(III) complexes are very stable.arrow_forwardMnO2 acts as an oxidant in the chlorine synthesis reaction.arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

