
Anatomy & Physiology
5th Edition
ISBN: 9780321861580
Author: Marieb, Elaine N.
Publisher: Pearson College Div
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 25, Problem 12RQ
The pH of blood varies directly with (a) HCO3–, (b) 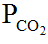 , (c) H+, (d) none of the above.
, (c) H+, (d) none of the above.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
From your previous experiment, you found that this enhancer activates stripe 2 of eve expression. When you sequence this enhancer you find several binding sites for the gap gene, Giant. To test how Giant interacts with eve, you decide to remove all of the Giant binding sites from the eve enhancer. What results do you expect to see with respect to eve expression?
What experiment could you do to see if the maternal gene, bicoid, is sufficient to form anterior fates?
You’re curious about the effect that gap genes have on the pair-rule gene, evenskipped (eve), so you isolate and sequence each of the eve enhancers. You’re particularly interested in one of the enhancers, which is just upstream of the eve gene.
Describe an experimental technique you would use to find out where this particular eve enhancer is active.
Chapter 25 Solutions
Anatomy & Physiology
Ch. 25 - Which do you have more of, extracellular or...Ch. 25 - What is the major cation in the ECF? In ICF? What...Ch. 25 - If you eat salty pretzels without drinking, what...Ch. 25 - Prob. 4CYUCh. 25 - ADH, by itself, cannot reduce an increase in...Ch. 25 - For each of the following, state whether it might...Ch. 25 - Jacob has Addisons disease (insufficient...Ch. 25 - Prob. 8CYUCh. 25 - Prob. 9CYUCh. 25 - Define acidemia and alkalemia.
Ch. 25 - Prob. 11CYUCh. 25 - What are the bodys three major chemical buffer...Ch. 25 - Joanne, a diabetic patient, is at the emergency...Ch. 25 - Reabsorption of HCO3 is always tied to the...Ch. 25 - Prob. 15CYUCh. 25 - Prob. 16CYUCh. 25 - Which two abnormalities in plasma are key features...Ch. 25 - How do the kidneys compensate for respiratory...Ch. 25 - Body water content is greatest in (a) infants, (b)...Ch. 25 - Potassium, magnesium, and phosphate ions are the...Ch. 25 - Sodium balance is regulated primarily by control...Ch. 25 - Prob. 4RQCh. 25 - Two main substances regulated by the influence of...Ch. 25 - Two substances regulated by parathyroid hormone.Ch. 25 - Two substances secreted into the proximal...Ch. 25 - Prob. 8RQCh. 25 - Prob. 9RQCh. 25 - Prob. 10RQCh. 25 - Prob. 11RQCh. 25 - The pH of blood varies directly with (a) HCO3, (b)...Ch. 25 - In an individual with metabolic acidosis, a clue...Ch. 25 - Name the body fluid compartments, noting their...Ch. 25 - Prob. 15RQCh. 25 - Prob. 16RQCh. 25 - Prob. 17RQCh. 25 - Prob. 18RQCh. 25 - Explain how the chemical buffer systems resist...Ch. 25 - Explain the relationship of the following to renal...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- For short answer questions, write your answers on the line provided. To the right is the mRNA codon table to use as needed throughout the exam. First letter U บบบ U CA UUCPhe UUA UCU Phe UCC UUG Leu CUU UAU. G U UAC TV UGCys UAA Stop UGA Stop A UAG Stop UGG Trp Ser UCA UCG CCU] 0 CUC CUA CCC CAC CAU His CGU CGC Leu Pro CCA CAA Gin CGA Arg CUG CCG CAG CGG AUU ACU AAU T AUC lle A 1 ACC Thr AUA ACA AUG Mot ACG AGG Arg GUU GCU GUC GCC G Val Ala GAC Asp GGU GGC GUA GUG GCA GCG GAA GGA Gly Glu GAGJ GGG AACASH AGU Ser AAA1 AAG Lys GAU AGA CAL CALUCAO CAO G Third letter 1. (+7) Use the table below to answer the questions; use the codon table above to assist you. The promoter sequence of DNA is on the LEFT. You do not need to fill in the entire table. Assume we are in the middle of a gene sequence (no need to find a start codon). DNA 1 DNA 2 mRNA tRNA Polypeptide C Val G C. T A C a. On which strand of DNA is the template strand (DNA 1 or 2)?_ b. On which side of the mRNA is the 5' end (left or…arrow_forward3. (6 pts) Fill in the boxes according to the directions on the right. Structure R-C R-COOH OH R-OH i R-CO-R' R R-PO4 R-CH3 C. 0 R' R-O-P-OH 1 OH H R-C-H R-N' I- H H R-NH₂ \H Name Propertiesarrow_forward4. (6 pts) Use the molecule below to answer these questions and identify the side chains and ends. Please use tidy boxes to indicate parts and write the letter labels within that box. a. How many monomer subunits are shown? b. Box a Polar but non-ionizable side chain and label P c. Box a Basic Polar side chain and label BP d. Box the carboxyl group at the end of the polypeptide and label with letter C (C-terminus) H H OHHO H H 0 HHO H-N-CC-N-C-C N-C-C-N-GC-OH I H-C-H CH2 CH2 CH2 H3C-C+H CH2 CH2 OH CH CH₂ C=O OH CH2 NH2arrow_forward
- please draw in what the steps are given. Thank you!arrow_forwardplease draw in and fill out the empty slots from image below. thank you!arrow_forwardThere is a species of eagle, which lives in a tropical forest in Brazil. The alula pattern of its wings is determined by a single autosomal gene with four alleles that exhibit an unknown hierarchy of dominance. Genetic testing shows that individuals 1-1, 11-4, 11-7, III-1, and III-4 are each homozygous. How many possible genotypes among checkered eagles in the population?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning



Human Heredity: Principles and Issues (MindTap Co...
Biology
ISBN:9781305251052
Author:Michael Cummings
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning
Biochemical Tests-Part 1; Author: Southern Stacker;https://www.youtube.com/watch?v=a-i9vANfQWQ;License: Standard Youtube License