
(a)
Interpretation:
For each of the given bifunctional compounds, the cyclic hemiacetal formed when treated with aqueous acid should be drawn.
Concept introduction:
- Hemiacetal are formed for the compound having both hydroxyl group and
aldehyde group in acidic condition. - The oxygen atom in the –OH group of compound attacks the carbonyl group of the same compound, thereby a ring is formed by the carbon atoms and oxygen atom of –OH group with incorporating into the ring.
To draw: the hemiacetal formed for the given molecule when treated with aqueous acid.
(b)
Interpretation:
For each of the given bifunctional compounds, the cyclic hemiacetal formed when treated with aqueous acid should be drawn.
Concept introduction:
- Hemiacetal are formed for the compound having both hydroxyl group and aldehyde group in acidic condition.
- The oxygen atom in the –OH group of compound attacks the carbonyl group of the same compound, thereby a ring is formed by the carbon atoms and oxygen atom of –OH group with incorporating into the ring.
To draw: the hemiacetal formed for the given molecule when treated with aqueous acid.
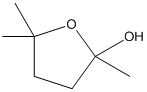
(c)
Interpretation:
For each of the given bifunctional compounds, the cyclic hemiacetal formed when treated with aqueous acid should be drawn.
Concept introduction:
- Hemiacetal are formed for the compound having both hydroxyl group and aldehyde group in acidic condition.
- The oxygen atom in the –OH group of compound attacks the carbonyl group of the same compound, thereby a ring is formed by the carbon atoms and oxygen atom of –OH group with incorporating into the ring.
To draw: the hemiacetal formed for the given molecule when treated with aqueous acid.
(d)
Interpretation:
For each of the given bifunctional compounds, the cyclic hemiacetal formed when treated with aqueous acid should be drawn.
Concept introduction:
- Hemiacetal are formed for the compound having both hydroxyl group and aldehyde group in acidic condition.
- The oxygen atom in the –OH group of compound attacks the carbonyl group of the same compound, thereby a ring is formed by the carbon atoms and oxygen atom of –OH group with incorporating into the ring.
To draw: the hemiacetal formed for the given molecule when treated with aqueous acid.
Trending nowThis is a popular solution!

Chapter 24 Solutions
ORGANIC CHEMISTRY (LL) W/ACCESS
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





