
(a)
Interpretation:
For the four stereo isomeric aldotetroses, the pair of enantiomers and stereo descriptors is needed to be drawn.
Concept introduction:
- Aldoses are monosaccharides, which contains
Aldehyde group. - The naming of monosaccharides,
- Prefix: aldo or keto indicates whether the monosaccharide is containing aldehyde or
ketone group. - Suffix: ose indicates the carbohydrates
- Root word: indicate the number of carbons.
- Prefix: aldo or keto indicates whether the monosaccharide is containing aldehyde or
- The stereo-descriptor used for monosaccharides is D or L. it is based on the dextrorotatory glyceraldehyde or levorotatory glyceraldehyde.
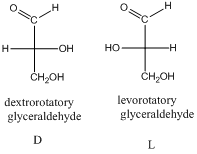
The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Enantiomers are non-super imposable mirror images of each other.
To determine: the structures of pair of enantiomers of stereo isomeric aldotetroses.
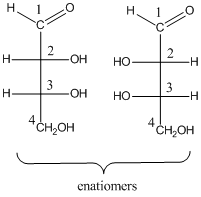
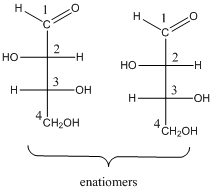
(b)
Interpretation:
For the four stereo isomeric aldotetroses, the pair of enantiomers and stereo descriptors is needed to be drawn.
Concept introduction:
- Aldoses are monosaccharides, which contains Aldehyde group.
- The naming of monosaccharides,
- Prefix: aldo or keto indicates whether the monosaccharide is containing aldehyde or ketone group.
- Suffix: ose indicates the carbohydrates
- Root word: indicate the number of carbons.
- The stereo-descriptor used for monosaccharides is D or L. it is based on the dextrorotatory glyceraldehyde or levorotatory glyceraldehyde.

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Enantiomers are non-super imposable mirror images of each other.
To identify: the D sugar and L sugar from the four structures of stereo isomeric aldotetroses.
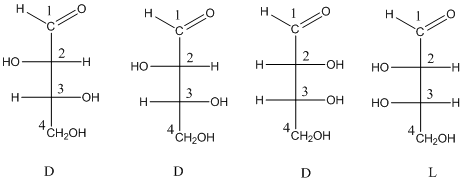
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- Starting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forwardIndicate the products obtained by treating benzotriazole with dimethyl sulfate or methyl iodide in a basic medium.arrow_forward
- What is the significance of selecting a "representative" sample for chemical analysis, and how does this practice ensure accurate and reliable results with respect to chemical analyses?arrow_forwardIdentify and provide an explanation of the differences between homogeneous and heterogeneous sampling in the context of sampling methods.arrow_forwardГ C-RSA CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.0 b.092 0.797 1.088 1.813 C-RSA CHROMATOPAC CH=1 Report No. =13 ** CALCULATION REPORT ** DATA=1: @CHRM1.000 11/03/05 08:09:52 CH PKNO TIME 1 2 0.797 3 1.088 4 1.813 AREA 1508566 4625442 2180060 HEIGHT 207739 701206 V 287554 V MK IDNO CONC NAME 18.1447 55.6339 26.2213 TOTAL 8314067 1196500 100 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0. 0 087 337. 0.841 1.150 C-R8A CHROMATOPAC CH=1 Report No. =14 DATA=1: @CHRM1.000 11/03/05 08:12:40 ** CALCULATION REPORT ** CH PKNO TIME AREA 1 3 0.841 1099933 41.15 4039778 HEIGHT MK IDNO 170372 649997¯¯¯ CONC NAME 21.4007 78.5993 TOTAL 5139711 820369 100 3 C-R8A CHROMATOPAC CH=1 DATA 1: @CHRM1.C00 ATTEN=10 SPEED= 10.0 0.100 0:652 5.856 3 1.165 C-RSA CHROMATOPAC CH-1 Report No. =15 DATA=1: @CHRM1.000 11/03/05 08:15:26 ** CALCULATION REPORT ** CH PKNO TIME AREA HEIGHT MK IDNO CONC NAME 1 3 3 0.856 4 1.165 TOTAL 1253386 4838738 175481 708024 V 20.5739 79.4261 6092124…arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate please.arrow_forwardRelative Abundance 20- Problems 501 (b) The infrared spectrum has a medium-intensity peak at about 1650 cm. There is also a C-H out-of-plane bending peak near 880 cm. 100- 80- 56 41 69 M(84) LL 15 20 25 30 35 55 60 65 70 75 80 85 90 m/zarrow_forwardPolyethylene furanoate is a polymer made from plant-based sources; it is used for packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





