
(a)
Interpretation:
The HOMO of one reactant and LUMO of the other reactant in the given reaction are to be drawn assuming one of the reactants is in its lowest excited state.
Concept introduction:
The bonding or antibonding character of an MO is determined by the number of constructive and destructive interactions. When the number of constructive (same phase) interactions exceed the number of destructive interactions, the MO is bonding. If the number of destructive interactions is larger, the MO is antibonding.
The highest energy bonding MO is the last one that contains electrons and is called the Highest Energy Molecular orbital (HOMO). The antibonding MO immediately above it in is the Lowest Energy Unoccupied Molecular Orbital, called LUMO.
For the molecule in its lowest excited state, one of the electrons from the ground state HOMO is promoted to the ground state LUMO. Therefore, the LUMO of the ground state, the first of the antibonding MOs now becomes the HOMO, and the LUMO is the second antibonding MO.
Answer to Problem 24.62P
The HOMO of one reactant and the LUMO of the other reactant for the three reactions are
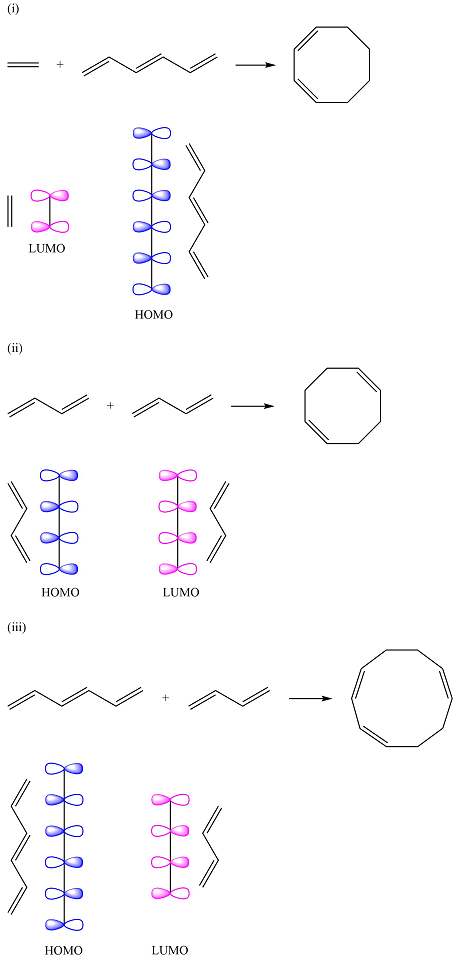
Explanation of Solution
Reaction (i) is
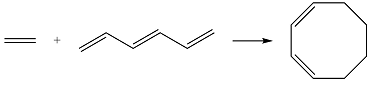
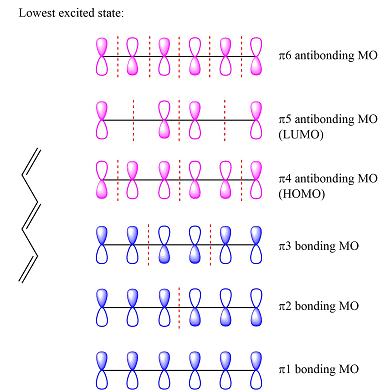
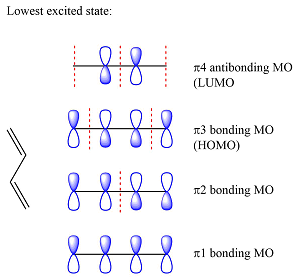
Assuming the larger reactant to be the diene in its lowest excited state, i.e., the donor molecule in the Diels-Alder reaction, the HOMO of was determined by considering the different interactions between the six p orbitals of the carbons. The possible interactions are
With three
The smaller of the reactants has only two carbons and one
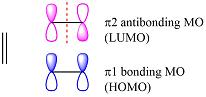
Thus, the HOMO and LUMO for this reaction are:
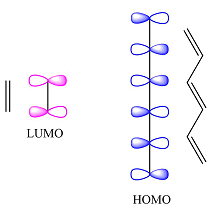
Reaction (ii) is
The reactants are the same. They both have four carbons, and therefore, p orbitals. The interaction of these will result in four
The MOs of the other molecule remain the same as in the ground state
Thus, the HOMO and LUMO in this case are
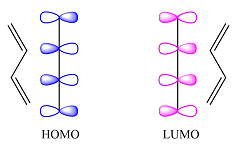
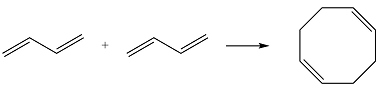
Reaction (iii) is
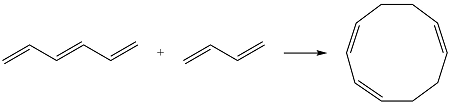
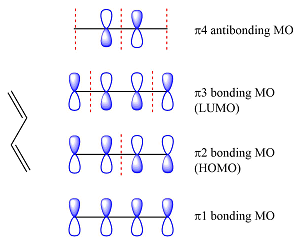
The first reactant has six carbons that contribute six p orbitals. The six
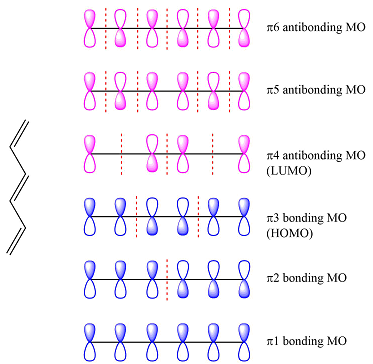
Assuming this molecule is in the lowest excited state, one of the electrons from its ground state HOMO (
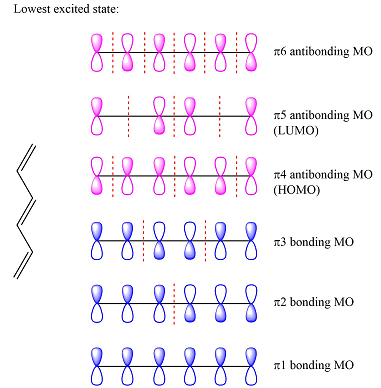
The second reactant has four carbons that contribute four p orbitals. The four
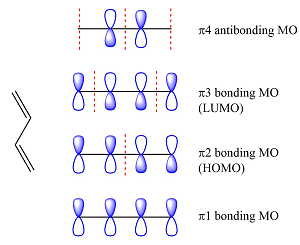
Again, assuming that the larger molecule acts as the donor (diene) and the smaller as the acceptor (dienophile) molecule, the HOMO and LUMO that interact in the Diels-Alder reaction are
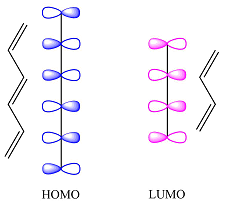
The
(b)
Interpretation:
The HOMO-LUMO interaction between the two reactants is to be drawn and if it will result in a thermally allowed or forbidden reaction is to be determined.
Concept introduction:
Diels-Alder reaction is a concerted reaction between a diene and a dienophile that produces a cyclic compound. It replaces two
In terms of MO theory, the reaction requires that the overlapping orbitals of the two molecules be in phase so that constructive interference occurs.
The electron flow starts with the diene donating a pair of
Answer to Problem 24.62P
The HOMO-LUMO interactions for the three reactions are:
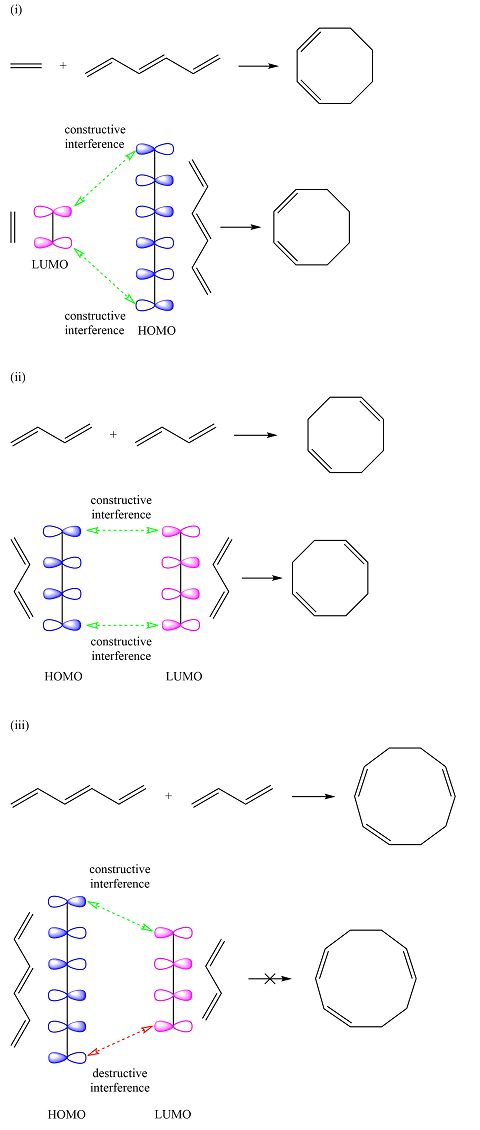
Reactions (i) and (ii) are thermally allowed reactions. Reaction (iii) is a thermally forbidden reaction.
Explanation of Solution
Reaction (i) is

Based on the HOMO and LUMO from part (a), the HOMO-LUMO interaction for this reaction is
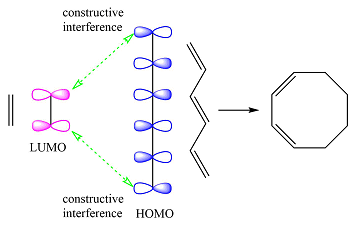
Both interactions are constructive. The net result is, therefore, a bonding interaction. Therefore, the reaction is thermally allowed.
Reaction (ii) is
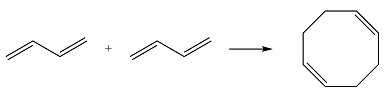
Based on the HOMO and LUMO from part (a), the HOMO-LUMO interaction for this reaction is
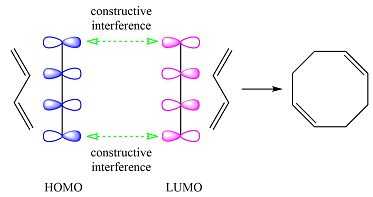
Both interactions are constructive. The net result is, therefore, a bonding interaction. Therefore, the reaction is thermally allowed.
Reaction (iii) is
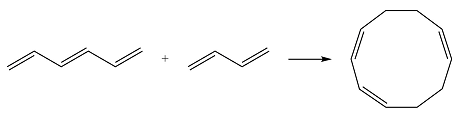
Based on the HOMO and LUMO from part (a), the HOMO-LUMO interaction for this reaction is
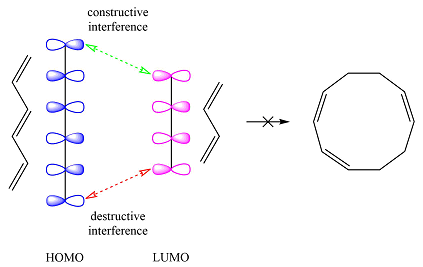
One of the interactions is constructive while the other is destructive, i.e., the net interaction is zero. Therefore, this reaction is thermally forbidden.
The HOMO-LUMO interaction between the reactants is determined based on the overlap and phases of the donor and acceptor MOs respectively and the feasibility of the reaction determined from the overall character of the interaction.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry: Principles And Mechanisms
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forward
- Why doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forwardWhat is the mechanism for this?arrow_forward
- For questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forward
- What is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





