
Concept explainers
a)
Interpretation:
The structure of the
Concept introduction:
- The longest continuous ring of carbon atoms is identified. The prefix is added with ‘cyclo’.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
a)
Explanation of Solution
Given name: cyclopentane
Predict the longest continuous chain of carbon atoms:
The parent name is CYCLOPENTANE represent the continuous ring of carbon atoms contains five carbons. The Suffix ‘ane’ represents the structure contains only single bonds.
Predict substituents and its location:
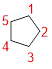
No prefix available before the parent name pentane, thus, the structure has no substituents located at any carbon atoms.
Therefore, the structure is

b)
Interpretation:
The structure of cis-2-butene has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
Geometric isomers of Alkenes:
Cis-isomer: When two particular atoms (identical group of atoms) are adjacent (on same side) to each other, the alkene is known as cis-isomer.
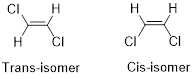
Trans-isomer: When two particular atoms (identical group of atoms) are opposite from each other, the alkene is known as trans-isomer.
b)
Explanation of Solution
Given name: cis-2-butene
Predict the longest continuous chain of carbon atoms:
The parent name is BUTENE represent the longest chain of carbon atoms contains four carbons. The Suffix ‘ene’ represents presence of double bond at C-2.
Predict substituents and its location:
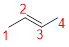
No prefix available before the parent name pentane, thus, the structure has no substituents located at any carbon atoms. The term ‘cis-’ indicates two hydrogen atoms are located adjacent to each other on same side.
Therefore, the structure is
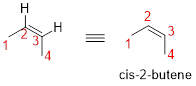
c)
Interpretation:
The structure of 2-hexanol has to be predicted.
Concept introduction:
IUPAC Nomenclature of alkanes:
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
c)
Explanation of Solution
Given name: 2-hexanol
Predict the longest continuous chain of carbon atoms:
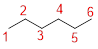
The parent name is HEXANOL represent the longest chain of carbon atoms contains six carbons. The Suffix ‘ol’ represents presence of hydroxy group –OH at C-2.
Predict substituents and its location:
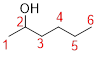
The prefix ‘2-‘represent the presence of hydroxy group at C-2.
Therefore, the structure is
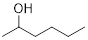
d)
Interpretation:
The structure of 1,4-dibromobenzene has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The parent compound of aromatic compound is Benzene.
- The substituent groups attached to the parent is identified. A substituent group contains group of atoms attached to the carbon atom of the aromatic ring.
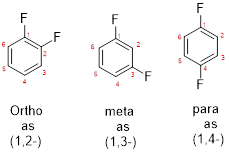
- While numbering the parent, the location of the second group relative to the first substituent uses prefixes like o-ortho (1,2-), m-meta (1,3-), and p-para (1,4) in disubstituted aromatic ring.
d)
Explanation of Solution
Given name: 1,4-dibromobenzene
Predict the parent structure:
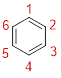
The parent name is BENZENE represent the continuous ring of carbon atoms contains six carbons with alternative double bonds.
Predict substituents and its location:
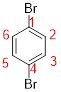
The prefix ‘1, 4-dibromo’ indicates the presence of two identical bromine atom located at C-1 and C-4.
Therefore, the structure is
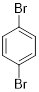
e)
Interpretation:
The structure of 2-butyne has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The longest continuous chain of carbon atoms is identified. The suffix is added replaced with ‘yne’.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
e)
Explanation of Solution
Given name: 2-butyne
Predict the longest continuous chain of carbon atoms:

The parent name is BUTYNE represent the longest chain of carbon atoms contains four carbons. The Suffix ‘yne’ represents the structure contains only one triple bonds at C-2.
Predict substituents and its location:

No prefix available before the parent name pentane, thus, the structure has no substituents located at any carbon atoms.
Therefore, the structure is

Want to see more full solutions like this?
Chapter 24 Solutions
CHEMISTRY (LL) W/CNCT >BI<
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





