
(a)
Interpretation:
Empirical formula of the given substance has to be predicted.
Concept introduction:
Steps to calculate empirical formula:
- Convert the mass of elements into moles.
- Divide each mole value by the smallest number of moles calculated.
- Round to the nearest whole number.
Number of moles = Molarity
(a)
Explanation of Solution
Calculate moles of each given elements:
This gives the formula
b)
Interpretation:
Does the substance behave as an ideal gas has to be predicted.
Concept introduction:
Ideal gas equation:
Boyle’s law: The pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
b)
Explanation of Solution
When temperature and amount of gas are constant, the product of pressure times volume is constant (Boyle’s law).
For given pressure and volume values,
If the number of moles and temperature are remains constant, then the product of pressure and volume should be same. If not then substances does not behave as an ideal gas.
As shown above none of the values are same. Hence, the substances do not behave as an ideal gas.
c)
Interpretation:
The molecular formula has to be predicted.
Concept introduction:
Steps to calculate empirical formula:
- Convert the mass of elements into moles.
- Divide each mole value by the smallest number of moles calculated.
- Round to the nearest whole number.
Number of moles = Molarity
c)
Explanation of Solution
Calculate moles of each given elements:
This gives the formula
Now, let’s calculate moles using the ideal gas equation, and then calculate the molar mass.
The formula mass of
d)
Interpretation:
Lewis structure of the molecule and its geometry has to be drawn and described.
Concept introduction:
Structural Isomerism: Structural Isomers are the structure of a molecule with same molecular formula but have different arrangements of bonds and atoms and position of double bond also changes from more substituted to less substituted or vice-versa.
Lewis structure: The bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Geometric isomers of
Cis-isomer: When two particular atoms (group of atoms) are adjacent to each other, the alkene is known as cis-isomer.
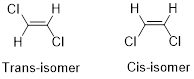
Trans-isomer: When two particular atoms (group of atoms) are across from each other, the alkene is known as trans-isomer.
d)
Explanation of Solution
Compound
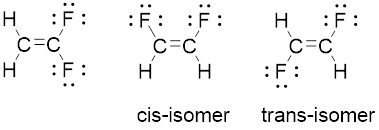
The geometry of each carbon is trigonal planar. Arrangement of two identical fluorine atoms on the same side adjacent to each other known as cis-isomer. And represnted opposite side to each other known as trans-isomer.
e)
Interpretation:
The systematic name of the structure has to be written.
Concept introduction:
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
Geometric isomers of Alkenes:
Cis-isomer: When two particular atoms (group of atoms) are adjacent to each other, the alkene is known as cis-isomer.
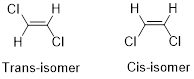
Trans-isomer: When two particular atoms (group of atoms) are across from each other, the alkene is known as trans-isomer.
e)
Explanation of Solution
Given name: cis-2-butene
Predict the longest continuous chain of carbon atoms:
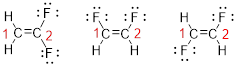
The parent name is ETHENE represent the longest chain of carbon atoms contains two carbons. The Suffix ‘ene’ represents presence of double bond at C-1.
Predict substituents and its location:
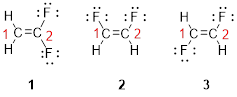
The first compound structure has two fluorine atoms located at carbon-1. Hence the name can be written as substituent followed by parent name; 2,2-difluoroethene.
The second compound structure has two fluorine atoms located at carbon-1and 2. The term ‘cis-’ indicates two fluorine atoms are located adjacent to each other on same side. Hence the name can be written as substituent followed by parent name; cis-1,2-difluoroethene.
The third compound structure has two fluorine atoms located at carbon-1and 2. The term ‘trans-’ indicates two fluorine atoms are located opposite to each other. Hence the name can be written as substituent followed by parent name; trans-1,2-difluoroethene.
Want to see more full solutions like this?
Chapter 24 Solutions
Chemistry
- → Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
- Identifying electron-donating and For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects NH2 ○ donating NO2 Explanation Check withdrawing no inductive effects Resonance Effects Overall Electron-Density ○ donating O withdrawing O no resonance effects O donating O withdrawing O donating withdrawing O no inductive effects Ono resonance effects O electron-rich electron-deficient O similar to benzene O electron-rich O electron-deficient O similar to benzene olo 18 Ar 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check Х (Choose one) OH (Choose one) OCH3 (Choose one) OH (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardAssign R or S to all the chiral centers in each compound drawn below porat bg 9 Br Brarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


