
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
4th Edition
ISBN: 9781119234722
Author: Klein
Publisher: JOHN WILEY+SONS,INC.-CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.3, Problem 2.3P
For each of the following compounds, determine whether or not you would expect its IR spectrum to exhibit a signal to the left of 3000 cm–1
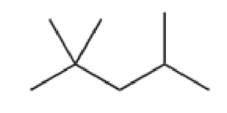
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which compound(s) will be fully deprotonated (>99%) by reaction with one molar
equivalent of sodium hydroxide?
I, II, III
I, ||
I, III
I only
II, III
SH
|
H3C-C=C-H
||
III
NH2
Will NBS (and heat or light) work for this reaction, or do we have to use Br2?
HAND DRAW
Chapter 2 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - The following compound has three carbonyl groups....Ch. 2.4 - Predict which of the following C=C bonds will...Ch. 2.4 - The C=C bond in the following compound produces an...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...
Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.6 - Prob. 2.22P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
What type of cut would separate the brain into anterior and posterior parts?
Anatomy & Physiology (6th Edition)
Explain how the behavior of homologous chromosomes in meiosis parallels Mendels law of segregation for autosoma...
Genetic Analysis: An Integrated Approach (3rd Edition)
Practice Problem 7.23
Write the structure of compound A, used in this synthesis of the perfume ingredient (Z)-j...
Organic Chemistry
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of the following organic reaction: Some important notes: Δ CN ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. ONO reaction. Click and drag to start drawing a structure.arrow_forwardThe following product was made from diethyl ketone and what other reagent(s)? £ HO 10 2-pentyne 1-butyne and NaNH2 ☐ 1-propanol ☐ pyridine butanal ☐ pentanoatearrow_forwardWhich pair of reagents will form the given product? OH X + Y a. CH3 b. CH2CH3 ༧་་ C. CH3- CH2CH3 d.o6.(རི॰ e. CH3 OCH2CH3 -MgBr f. CH3-MgBr g. CH3CH2-MgBr -C-CH3 CH2CH3arrow_forward
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY