
(a)
Interpretation: Which fatty acid would most contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
Concept Used:
Oil consists of large number of unsaturated fatty acids, whereas fats consists of large number of saturated fatty acid.
Also, saturated fatty acid do not have any carbon-carbon double bond, whereas unsaturated fatty acid consists of carbon-carbon double bond.
Unsaturated fatty acids which have more than one carbon-carbon double bond are referred to as polyunsaturated fatty acid.
To determine:
Find out if fatty acid 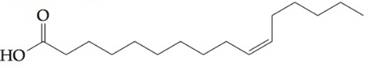 would contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
would contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
(b)
Interpretation: Which fatty acid would most contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
Concept Used:
Oil consists of large number of unsaturated fatty acids, whereas fats consists of large number of saturated fatty acid.
Also, saturated fatty acid do not have any carbon-carbon double bond, whereas unsaturated fatty acid consists of carbon-carbon double bond.
Unsaturated fatty acids which have more than one carbon-carbon double bond are referred to as polyunsaturated fatty acid.
To determine: Find out if fatty acid 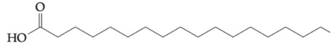 would contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
would contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
(c)
Interpretation: Which fatty acid would most contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
Concept Used:
Oil consists of large number of unsaturated fatty acids, whereas fats consists of large number of saturated fatty acid.
Also, saturated fatty acid do not have any carbon-carbon double bond, whereas unsaturated fatty acid consists of carbon-carbon double bond.
Unsaturated fatty acids which have more than one carbon-carbon double bond are referred to as polyunsaturated fatty acid
To determine: Find out if fatty acid 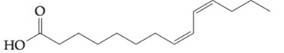 would contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
would contribute to a triacylglyceride being oil, rather than a fat, at a room temperature.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
- Use diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward15) Create Lewis structure Br Brarrow_forward
- LIOT S How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate? View Rubricarrow_forwardSteps and explantions pleasearrow_forwardMatch the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forward
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





