
Concept explainers
(a)
Interpretation:
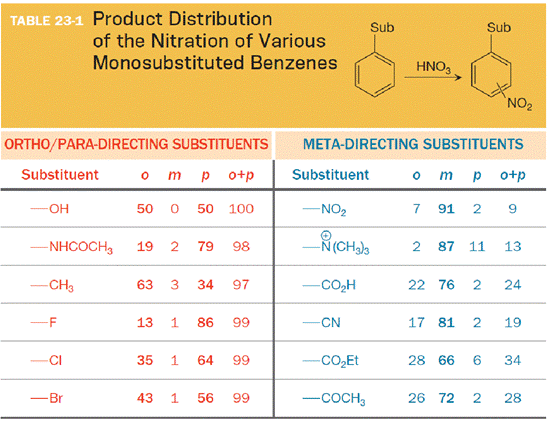
All lone pairs to the atom at the point of attachment for every substituent in Table 23-1 are to be added. Also, out of the two columns, the column (ortho/para- or meta directing substituents) in which these substituents can be found is to be given.
Concept introduction:
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
(b)
Interpretation:
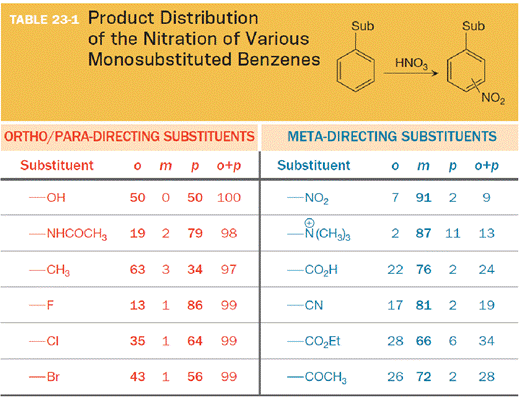
The substituent in the column attached by an atom that has no lone pairs of electrons is to be shown. Also any electronegative atoms at or near the point of attachment are to be shown.
Concept introduction:
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
(c)
Interpretation:
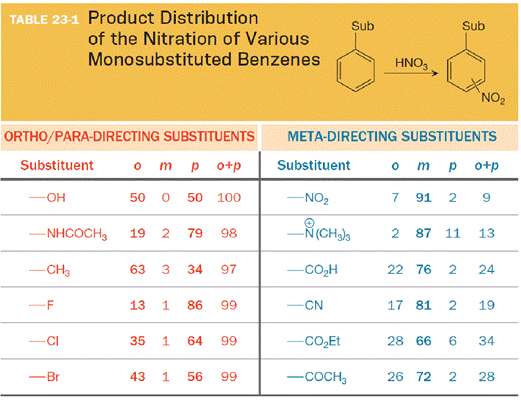
In the other column, electronegative atoms at or near the point of attachment are to be found.
Concept introduction:
The substituent on the benzene ring prior to substitution dictates the regiochemistry of the reaction. Some substituents are designated as ortho/para directors because they lead to product mixtures consisting primarily of the ortho- and para-disubstituted products. Other substituents are designated as meta directors because they favor the formation of the meta-disubstituted product. The results from nitration reactions are summarized in Table 23-1:
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry: Principles And Mechanisms (second Edition)
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
