
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260826791
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22.6, Problem 21P
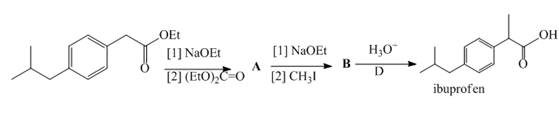
Two steps in a synthesis of the analgesic ibuprofen, the chapter-opening molecule, include a carbonyl condensation reaction, followed by an alkylation reaction. Identify intermediates A and B in the synthesis of ibuprofen.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.
Electrochemistry. State the difference between E and E0.
In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?
Chapter 22 Solutions
ORGANIC CHEMISTRY
Ch. 22.1 - Prob. 1PCh. 22.1 - Prob. 2PCh. 22.1 - Problem 24.3
What unsaturated carbonyl compound is...Ch. 22.1 - Prob. 4PCh. 22.1 - Prob. 5PCh. 22.2 - Prob. 6PCh. 22.2 - Problem 24.7
Draw the products formed in each...Ch. 22.4 - Prob. 15PCh. 22.4 - Prob. 16PCh. 22.5 - Problem 24.16
What ester is formed when each...
Ch. 22.6 - Prob. 18PCh. 22.6 - Prob. 19PCh. 22.6 -
Draw the products of each reaction.
a. b.
Ch. 22.6 - Problem 24.20
Two steps in a synthesis of the...Ch. 22.7 - Prob. 22PCh. 22.8 - Problem 24.22
Which of the following compounds can...Ch. 22.8 - Prob. 24PCh. 22.8 - Problem 24.24
What starting materials are needed...Ch. 22 - Prob. 29PCh. 22 - 24.29 What steps are needed to convert A to B?
Ch. 22 - Prob. 31PCh. 22 - 24.31 Draw the product formed in each directed...Ch. 22 - Prob. 33PCh. 22 - 24.33 What starting materials are needed to...Ch. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - 24.36 Identify the structures of C and D in the...Ch. 22 - Prob. 38PCh. 22 - Prob. 39PCh. 22 - 24.39 Draw the product formed from a Claisen...Ch. 22 - Prob. 41PCh. 22 - 24.41 Even though B contains three ester groups, a...Ch. 22 - Prob. 43PCh. 22 - Prob. 44PCh. 22 - 24.44 Vetivone is isolated from vetiver, a...Ch. 22 - Draw the product of each Robinson annulation from...Ch. 22 - Prob. 50PCh. 22 - Prob. 51PCh. 22 - 24.52 Draw a stepwise mechanism for the following...Ch. 22 - Prob. 53PCh. 22 - Prob. 54PCh. 22 - Prob. 55PCh. 22 - Prob. 56PCh. 22 - Prob. 57PCh. 22 - Prob. 58PCh. 22 - Prob. 59PCh. 22 - 24.62 Devise a synthesis of each compound from ,...Ch. 22 - Prob. 63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forwardIf the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forward
- Given the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forwardIndicate the functions that salt bridges have in batteries.arrow_forwardIn the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.arrow_forward
- Write the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?arrow_forwardState the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forward
- Easily differentiate between electrochemical potential and Galvani potential.arrow_forwardConstruct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY