
COLLEGE PHYSICS,AP EDITION >NASTA ED.<
4th Edition
ISBN: 9780134779218
Author: Knight
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 9CQ
- a. Which direction—clockwise or counterclockwise—does an electron travel through the wire in Figure Q22.9? Explain.
Figure Q22.Q
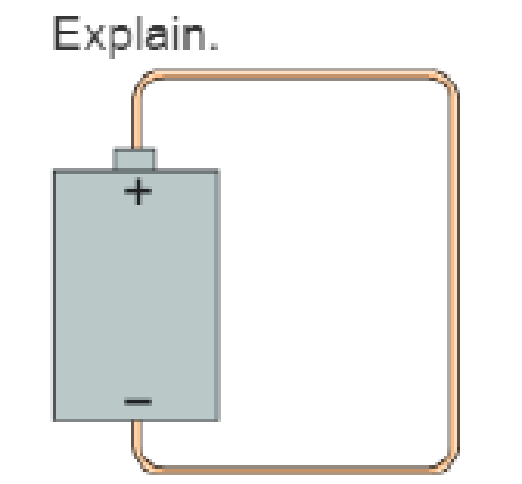
- b. Does an electron's electric potential energy increase, decrease, or stay the same as it moves through the wire? Explain.
- c. If you answered “decrease” in part b, where does the energy go? If you answered “increase” in part b, where does the energy come from?
- d. Which way—up or down—does an electron move through the battery? Explain.
- e. Does an electron's electric potential energy increase, decrease, or stay the same as it moves through the battery? Explain.
- f. If you answered “decrease” in part e, where does the energy go? If you answered “increase” in part e, where does the energy come from?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. Imagine you have a system in which you have 54 grams of ice. You can melt this
ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly
into a balloon held at a pressure of 0.250 bar. Here are some facts about water you
may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0
C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the
enthalpy of fusion of solid water is 333.55 J/gram.
A. How much energy does the ice absorb as heat when it melts?
B. How much work is involved in melting the ice?
C. What is the total change in energy for melting the ice?
D. What is the enthalpy change for melting the ice?
E. What is the entropy change for melting the ice?
F. What is the change in Helmholtz energy for melting the ice?
G. What is the change in Gibbs energy for melting the ice?
In the figure Q = 5.7 nC and all other quantities are accurate to 2 significant figures. What is the magnitude of the force on the charge Q? (k = 1/4πε 0 = 8.99 × 109 N · m2/C2)
Now add a fourth charged particle, particle 3, with positive charge q3, fixed in the yz-plane at (0,d2,d2). What is the net force F→ on particle 0 due solely to this charge? Express your answer (a vector) using k, q0, q3, d2, i^, j^, and k^. Include only the force caused by particle 3.
Chapter 22 Solutions
COLLEGE PHYSICS,AP EDITION >NASTA ED.<
Ch. 22 - Prob. 2CQCh. 22 - Prob. 3CQCh. 22 - Prob. 4CQCh. 22 - All wires in Figure Q22.519 are made of the same...Ch. 22 - A wire carries a 4 A current. What is the current...Ch. 22 - Prob. 7CQCh. 22 - Cells in the nervous system have a potential...Ch. 22 - a. Which directionclockwise or...Ch. 22 - Prob. 10CQCh. 22 - Prob. 11CQ
Ch. 22 - The two circuits in Figure Q22.12 use identical...Ch. 22 - The two circuits in Figure Q22.13 use identical...Ch. 22 - Prob. 14CQCh. 22 - Rank in order, from largest to smallest, the...Ch. 22 - The circuit in Figure Q22.16 has three batteries...Ch. 22 - Prob. 17CQCh. 22 - Prob. 18CQCh. 22 - Over time, atoms boil off the hot filament in an...Ch. 22 - Prob. 20CQCh. 22 - A 100 W lightbulb is brighter than a 60 W...Ch. 22 - Lightbulbs are typically rated by their power...Ch. 22 - Lightbulbs are typically rated by their power...Ch. 22 - A copper wire is stretched so that its length...Ch. 22 - The potential difference across a length of wire...Ch. 22 - Prob. 26MCQCh. 22 - A resistor connected to a 3.0 V battery dissipates...Ch. 22 - If a 1.5 V battery stores 5.0 kJ of energy (a...Ch. 22 - Figure Q22.29 shows a side view of a wire of...Ch. 22 - A person gains weight by adding fatand therefore...Ch. 22 - Prob. 31MCQCh. 22 - The current in an electric hair dryer is 10 A. How...Ch. 22 - Prob. 2PCh. 22 - Three wires meet at a junction. Wire 1 has a...Ch. 22 - When a nerve cell depolarizes, charge is...Ch. 22 - A wire carries a 15 A current. How many electrons...Ch. 22 - In a typical lightning strike, 2.5 C flows from...Ch. 22 - Prob. 7PCh. 22 - In an ionic solution, 5.0 1015 positive ions with...Ch. 22 - Prob. 9PCh. 22 - What are the values of currents IB and IC in...Ch. 22 - The currents through several segments of a wire...Ch. 22 - How much electric potential energy does 1.0 C of...Ch. 22 - What is the emf of a battery that increases the...Ch. 22 - A 9.0 V battery supplies a 2.5 mA current to a...Ch. 22 - Prob. 16PCh. 22 - An electric catfish can generate a significant...Ch. 22 - A Wire with resistance R is connected to the...Ch. 22 - Wires 1 and 2 are made of the same metal. Wire 2...Ch. 22 - Prob. 20PCh. 22 - Resistivity measurements on the leaves of corn...Ch. 22 - Prob. 22PCh. 22 - A motorcyclist is making an electric vest that,...Ch. 22 - Prob. 24PCh. 22 - A 3.0 V potential difference is applied between...Ch. 22 - Prob. 26PCh. 22 - Prob. 27PCh. 22 - Prob. 28PCh. 22 - Figure P22.29 shows the...Ch. 22 - Figure P22.30 is a...Ch. 22 - In Example 22.6 the length of a 60 W, 240 ...Ch. 22 - The electric field inside a 30-cm-long copper wire...Ch. 22 - A copper wire is 1.0 mm in diameter and carries a...Ch. 22 - Two identical lightbulbs are connected in series...Ch. 22 - Prob. 35PCh. 22 - a. What is the resistance of a 1500 W (120 V) hair...Ch. 22 - Prob. 37PCh. 22 - A 70 W electric blanket runs at 18 V. a. What is...Ch. 22 - A 60-cm-long heating wire is connected to a 120 V...Ch. 22 - An electric eel develops a potential difference of...Ch. 22 - Prob. 41PCh. 22 - A 3.0 V battery powers a flashlight bulb that has...Ch. 22 - A heating element in a toaster dissipates 900 W...Ch. 22 - Prob. 44GPCh. 22 - Prob. 45GPCh. 22 - The hot dog cooker described in the chapter heats...Ch. 22 - Air isnt a perfect electric insulator, but it has...Ch. 22 - The biochemistry that takes place inside cells...Ch. 22 - High-resolution measurements have shown that an...Ch. 22 - When an ion channel opens in a cell wall (see...Ch. 22 - The total charge a battery can supply is rated in...Ch. 22 - Prob. 52GPCh. 22 - The heating element of a simple heater consists of...Ch. 22 - Variations in the resistivity of blood can give...Ch. 22 - A 40 W (120 V) lightbulb has a tungsten filament...Ch. 22 - Prob. 56GPCh. 22 - When the starter motor on a car is engaged, there...Ch. 22 - Prob. 58GPCh. 22 - The two segments of the wire in Figure P22.59 have...Ch. 22 - Prob. 60GPCh. 22 - Prob. 61GPCh. 22 - Prob. 62GPCh. 22 - Prob. 63GPCh. 22 - Prob. 64GPCh. 22 - An immersion heater used to boil water for a...Ch. 22 - The graph in Figure P22.66 shows the current...Ch. 22 - Its possible to estimate the percentage of fat in...Ch. 22 - If you touch the two terminals of a power supply...Ch. 22 - The average resistivity of the human body (apart...Ch. 22 - MCAT-Style Passage Problems Lightbulb Failure...Ch. 22 - MCAT-Style Passage Problems Lightbulb Failure...Ch. 22 - MCAT-Style Passage Problems Lightbulb Failure...Ch. 22 - MCAT-Style Passage Problems Lightbulb Failure...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4. Three groups of nonvascular plants are _______, ______, and _______. Three groups of seedless vascular plant...
Biology: Life on Earth (11th Edition)
Describe the 1H NMR spectrum you would expect for each of the following compounds, indicating the relative posi...
Organic Chemistry (8th Edition)
A wild-type fruit fly (heterozygous for gray body color and led eyes) is mated Willi a black fruit fly wltli pu...
Campbell Biology (11th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk (*) desi...
Cosmic Perspective Fundamentals
27. Consider the reaction.
Express the rate of the reaction in terms of the change in concentration of each of...
Chemistry: Structure and Properties (2nd Edition)
What is the difference between cellular respiration and external respiration?
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- For a tornadoes and hurricanes, which of the following is most critical? an alert a watch a warning a predictionarrow_forwardWhen a warm front advances up and over a cold front, what is it called? front inversion stationary front cold front occlusion warm front occlusionarrow_forward1) Consider two positively charged particles, one of charge q0 (particle 0) fixed at the origin, and another of charge q1 (particle 1) fixed on the y-axis at (0,d1,0). What is the net force F→ on particle 0 due to particle 1? Express your answer (a vector) using any or all of k, q0, q1, d1, i^, j^, and k^. 2) Now add a third, negatively charged, particle, whose charge is −q2− (particle 2). Particle 2 fixed on the y-axis at position (0,d2,0). What is the new net force on particle 0, from particle 1 and particle 2? Express your answer (a vector) using any or all of k, q0, q1, q2, d1, d2, i^, j^, and k^. 3) Particle 0 experiences a repulsion from particle 1 and an attraction toward particle 2. For certain values of d1 and d2, the repulsion and attraction should balance each other, resulting in no net force. For what ratio d1/d2 is there no net force on particle 0? Express your answer in terms of any or all of the following variables: k, q0, q1, q2.arrow_forward
- A 85 turn, 10.0 cm diameter coil rotates at an angular velocity of 8.00 rad/s in a 1.35 T field, starting with the normal of the plane of the coil perpendicular to the field. Assume that the positive max emf is reached first. (a) What (in V) is the peak emf? 7.17 V (b) At what time (in s) is the peak emf first reached? 0.196 S (c) At what time (in s) is the emf first at its most negative? 0.589 x s (d) What is the period (in s) of the AC voltage output? 0.785 Sarrow_forwardA bobsled starts at the top of a track as human runners sprint from rest and then jump into the sled. Assume they reach 40 km/h from rest after covering a distance of 50 m over flat ice. a. How much work do they do on themselves and the sled which they are pushing given the fact that there are two men of combined mass 185 kg and the sled with a mass of 200 kg? (If you haven't seen bobsledding, watch youtube to understand better what's going on.) b. After this start, the team races down the track and descends vertically by 200 m. At the finish line the sled crosses with a speed of 55 m/s. How much energy was lost to drag and friction along the way down after the men were in the sled?arrow_forwardFor what type of force is it not possible to define a potential energy expression?arrow_forward
- 10. Imagine you have a system in which you have 54 grams of ice. You can melt this ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly into a balloon held at a pressure of 0.250 bar. Here are some facts about water you may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0 C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the enthalpy of fusion of solid water is 333.55 J/gram.arrow_forwardConsider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the supercooled water freezes at -10°C and 1 atm. Useful data: Cp (ice) = 38 J mol-1 K-1 Cp (water) 75J mol −1 K -1 Afus H (0°C) 6026 J mol −1 Assume Cp (ice) and Cp (water) to be independent of temperature.arrow_forwardThe molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol. Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of AvapG at 75.0°C, 80.09°C, and 85.0°C. Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.arrow_forward
- 3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forwardPlease don't use Chatgpt will upvote and give handwritten solutionarrow_forward6. We used the constant volume heat capacity, Cv, when we talked about thermodynamic cycles. It acts as a proportionality constant between energy and temperature: dU = C₁dT. You can also define a heat capacity for constant pressure processes, Cp. You can think of enthalpy playing a similar role to energy, but for constant pressure processes δαρ C = (37) - Sup Ср ат P = ат Starting from the definition of enthalpy, H = U + PV, find the relationship between Cy and Cp for an ideal gas.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Electric Fields: Crash Course Physics #26; Author: CrashCourse;https://www.youtube.com/watch?v=mdulzEfQXDE;License: Standard YouTube License, CC-BY