
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
3rd Edition
ISBN: 9781119497479
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 94IP
Interpretation Introduction
Interpretation:
The expected major product for the given reaction has to be found.
Concept Introduction:
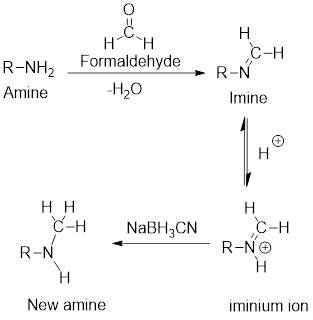
Reductive amination reaction:
It is the preparation of
Example:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Where are the chiral centers in this molecule? Also is this compound meso yes or no?
PLEASE HELP! URGENT!
Where are the chiral centers in this molecule? Also is this compound meso yes or no?
Chapter 22 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 22.2 - Prob. 1LTSCh. 22.2 - Prob. 1PTSCh. 22.2 - Prob. 2PTSCh. 22.2 - Prob. 3ATSCh. 22.3 - Prob. 4CCCh. 22.3 - Prob. 5CCCh. 22.3 - Prob. 6CCCh. 22.3 - Prob. 7CCCh. 22.3 - Prob. 8CCCh. 22.3 - Prob. 9CC
Ch. 22.4 - Prob. 10CCCh. 22.4 - Prob. 11CCCh. 22.5 - Prob. 2LTSCh. 22.5 - Prob. 12PTSCh. 22.5 - Prob. 13ATSCh. 22.6 - Prob. 3LTSCh. 22.6 - Prob. 14PTSCh. 22.6 - Prob. 15ATSCh. 22.7 - Prob. 4LTSCh. 22.7 - Prob. 16PTSCh. 22.7 - Prob. 17PTSCh. 22.7 - Prob. 18PTSCh. 22.7 - Prob. 19ATSCh. 22.8 - Prob. 20CCCh. 22.8 - Prob. 21CCCh. 22.8 - Prob. 22CCCh. 22.9 - Prob. 5LTSCh. 22.9 - Prob. 23PTSCh. 22.9 - Prob. 24ATSCh. 22.10 - Prob. 25CCCh. 22.11 - Prob. 26CCCh. 22.11 - Prob. 6LTSCh. 22.11 - Prob. 27PTSCh. 22.11 - Prob. 28ATSCh. 22.12 - Prob. 29CCCh. 22.12 - Prob. 30CCCh. 22.13 - Prob. 31CCCh. 22.13 - Prob. 32CCCh. 22 - Prob. 33PPCh. 22 - Prob. 34PPCh. 22 - Prob. 35PPCh. 22 - Prob. 36PPCh. 22 - Prob. 37PPCh. 22 - Prob. 38PPCh. 22 - Prob. 39PPCh. 22 - Prob. 40PPCh. 22 - Prob. 41PPCh. 22 - Prob. 42PPCh. 22 - Prob. 43PPCh. 22 - Prob. 44PPCh. 22 - Prob. 45PPCh. 22 - Prob. 46PPCh. 22 - Prob. 47PPCh. 22 - Prob. 48PPCh. 22 - Prob. 49PPCh. 22 - Prob. 50PPCh. 22 - Prob. 51PPCh. 22 - Prob. 52PPCh. 22 - Prob. 53PPCh. 22 - Prob. 54PPCh. 22 - Prob. 55PPCh. 22 - Prob. 56PPCh. 22 - Prob. 57PPCh. 22 - Prob. 58PPCh. 22 - Prob. 59PPCh. 22 - Prob. 60PPCh. 22 - Prob. 61PPCh. 22 - Prob. 62PPCh. 22 - Prob. 63PPCh. 22 - Prob. 64PPCh. 22 - Prob. 65PPCh. 22 - Prob. 66PPCh. 22 - Prob. 67PPCh. 22 - Prob. 68PPCh. 22 - Prob. 69PPCh. 22 - Prob. 70PPCh. 22 - Prob. 71PPCh. 22 - Prob. 72PPCh. 22 - Prob. 73IPCh. 22 - Prob. 74IPCh. 22 - Prob. 75IPCh. 22 - Prob. 76IPCh. 22 - Prob. 77IPCh. 22 - Prob. 78IPCh. 22 - Prob. 79IPCh. 22 - Prob. 80IPCh. 22 - Prob. 81IPCh. 22 - Prob. 82IPCh. 22 - Prob. 83IPCh. 22 - Prob. 84IPCh. 22 - Prob. 85IPCh. 22 - Prob. 86IPCh. 22 - Prob. 87IPCh. 22 - Prob. 88IPCh. 22 - Prob. 89IPCh. 22 - Prob. 90IPCh. 22 - Prob. 91IPCh. 22 - Prob. 92IPCh. 22 - Prob. 93IPCh. 22 - Prob. 94IPCh. 22 - Prob. 95IPCh. 22 - Prob. 96CPCh. 22 - Prob. 97CPCh. 22 - Prob. 98CPCh. 22 - Prob. 99CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License