
(a)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e)
Alkene on ozonolysis produces carbonyl compounds - f)
Alkyl halides with strong base gives alkene - g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions.
Aldehyde orketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primaryamines .
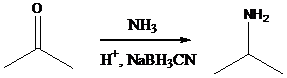
Using these concepts, we can transfer 1-hexanol into the given compounds.
(b)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e) Alkene on ozonolysis produces carbonyl compounds
- f) Alkyl halides with strong base gives alkene
- g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions. Aldehyde or ketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primary amines.
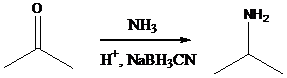
Using these concepts, we can transfer 1-hexanol into the given compounds.
(c)
Interpretation: Using a different type of reactions, 1-hexanol is to be prepared from hexyl amine, heptyl amine and pentyl amine
Concept Introduction: A number of transformations are used to prepare 1-hexanol. Some of them are listed as follows:
- a) Alcohol on treatment with phosphorous tribromide gives alkyl bromide
- b) Alkyl bromide in azide synthesis produces primary amine
- c) Alkyl halide on treatment with sodium cyanide gives alkyl cyanide
- d) Cyanide on reduction gives alkyl amine with an increment of one carbon atom skeleton
- e) Alkene on ozonolysis produces carbonyl compounds
- f) Alkyl halides with strong base gives alkene
- g) Sodium cyanoborohydride is a strong reducing agent than sodium borohydride. It reduces the carbonyl group into amine group in a rapid way. So, it is called as reductive amination reactions. Aldehyde or ketone group is reacted with ammonia in the presence of sodium cyanoborohydride as a reducing agent and a proton source in the reaction medium to produce primary amines.
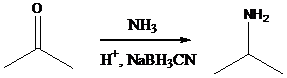
Using these concepts, we can transfer 1-hexanol into the given compounds.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEMISTRY (LL)-W/WILEYPLUS
- Name the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forward
- Provide the reagents for the following reactions.arrow_forwardIf I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





