
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
8th Edition
ISBN: 2818440043505
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 40P
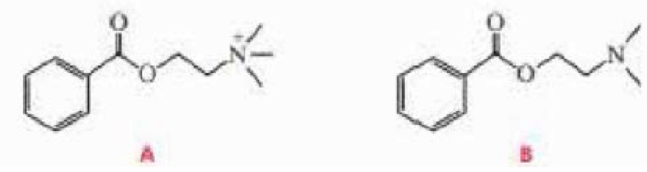
At pH = 12, the rate of hydrolysis of ester A is greater than the rate of hydrolysis of ester B. At pH = 8, the relative rates reverse (that is, ester B hydrolyzes faster than ester A). Explain these observations.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Topic: Photochemistry and Photophysics of Supramolecules
Two cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 C
With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.
Chapter 22 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
Ch. 22.2 - Compare each of the mechanisms listed here with...Ch. 22.2 - Prob. 3PCh. 22.2 - Prob. 4PCh. 22.3 - a. Draw the mechanism for the following reaction...Ch. 22.5 - Prob. 7PCh. 22.5 - Propose a mechanism for the Co2+ catalyzed...Ch. 22.6 - Prob. 9PCh. 22.7 - Prob. 10PCh. 22.7 - Prob. 12PCh. 22.7 - Prob. 13P
Ch. 22.9 - Which of the following amino acid side chains can...Ch. 22.9 - Which of the following C-terminal peptide bonds is...Ch. 22.9 - Carboxypeptidase A has esterase activity as well...Ch. 22.10 - Arginine and lysine side chains fit into trypsins...Ch. 22.10 - Explain why serine proteases do not catalyze...Ch. 22.11 - If H2 18O is used in the hydrolysis reaction...Ch. 22.11 - Draw the pH-activity profile for an enzyme that...Ch. 22.12 - The pHactivity profile for glucose-6-phosphate...Ch. 22.12 - Prob. 23PCh. 22.13 - Draw the mechanism for the hydroxide ion-catalyzed...Ch. 22.13 - What advantage does the enzyme gain by forming an...Ch. 22.13 - Prob. 26PCh. 22.13 - Prob. 27PCh. 22.13 - Aldolase shows no activity if it is incubated with...Ch. 22 - Which of the following parameters would be...Ch. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Indicate the type of catalysis that is occurring...Ch. 22 - The deuterium kinetic isotope effect (KH2O/KD2O)...Ch. 22 - Prob. 34PCh. 22 - Co2+ catalyzes the hydrolysis of the lactam shown...Ch. 22 - there are two kinds of aldolases. Class I...Ch. 22 - Prob. 37PCh. 22 - The hydrolysis of the ester shown here is...Ch. 22 - Prob. 39PCh. 22 - At pH = 12, the rate of hydrolysis of ester A is...Ch. 22 - 2-Acetoxycyclohexyl tosylate reacts with acetate...Ch. 22 - Proof that an imine was formed between aldolase...Ch. 22 - Prob. 43PCh. 22 - a. Explain why the alkyl halide shown here reacts...Ch. 22 - Triosephosphate isomerase (TIM) catalyzes the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License