
Concept explainers
(a)
Interpretation: The crystal field splitting of the given structures to be interpreted.
Concept Introduction:
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
To Identify: The crystal field splitting of the given structures to be interpreted.
(a)
Explanation of Solution
Interpret the reason.
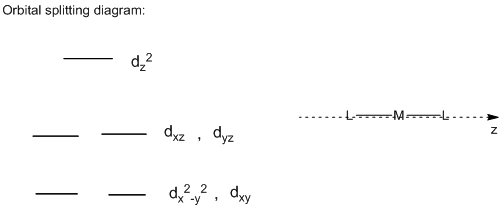
Orbital Splitting diagram of linear complex:
In the linear structure, the ligands which are pointing directly towards the z-axis, the
(b)
Interpretation: The crystal field splitting of the given structures to be interpreted.
Concept Introduction:
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
To Identify: The crystal field splitting of the given structures to be interpreted.
(b)
Explanation of Solution
Interpret the reason.
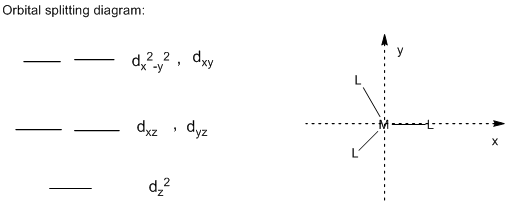
Orbital Splitting diagram of trigonal complex:
In the trigonal structure, the ligands which are pointing directly towards the x-axis and y-axis, the
(c)
Interpretation: The crystal field splitting of the given structures to be interpreted.
Concept Introduction:
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
To Identify: The crystal field splitting of the given structures to be interpreted.
(c)
Explanation of Solution
Interpret the reason.
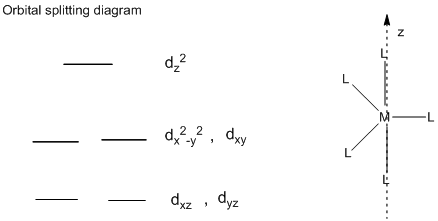
Orbital Splitting diagram of trigonal bipyramidal complex:
In the trigonal bipyramidal structure, the ligands which are pointing directly towards the z-axis, the
Want to see more full solutions like this?
Chapter 22 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





