
Concept explainers
(a)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(a)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion

The complex has square planar geometry.
Explanation of Solution
In the complex ion

The distribution of d-electrons as shown above correlates to that of square planar geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(b)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(b)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion

The complex has square planar geometry.
Explanation of Solution
In the complex ion
Atomic number of Cobalt is

The distribution of d-electrons as shown above correlates to that of square planar geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(c)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(c)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion
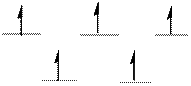
The complex has tetrahedral geometry.
Explanation of Solution
In the complex ion
Atomic number of Iron is

The distribution of d-electrons as shown above correlates to that of tetrahedral geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
(d)
Interpretation:
Distribution of d-electrons and the geometry of the complex ion
Concept Introduction:
Complex compounds exist in following geometries - tetrahedral, square planar, octahedral etc.
When ligands approach the metal ion the degeneracy in d-orbitals of the metal ion is destroyed and they split into two different energy levels. In case of tetrahedral complex, the
In case of square planar complex, the 5 d-orbitals split into following pattern and it is given below with increasing order of energy.
(d)
Answer to Problem 22.59QP
The d-electrons are distributed in the complex ion
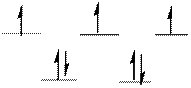
The complex has tetrahedral geometry.
Explanation of Solution
In the complex ion
Atomic number of Cobalt is

The distribution of d-electrons as shown above correlates to that of tetrahedral geometry. Hence the complex ion
Splitting of d-orbitals determines the geometry of the complex.
Want to see more full solutions like this?
Chapter 22 Solutions
Lab Manual Experiments in General Chemistry
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





