
Concept explainers
Give the structure corresponding to each name.
a. cyclohexyl propanoate
b. cyclohexanecarboxamide
c.
d. Vinyl acetate
e. Benzoic propanoic anhydride
f.
g. octyl butanoate
h. N, N-dibenzylformamide
(a)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is
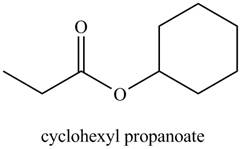
Explanation of Solution
The given name indicates that compound contains ester (name ends with –ate) as the functional group, and propane as the longest carbon chain with one cyclohexyl substitution as
Thus, the structure corresponding to the given name is,
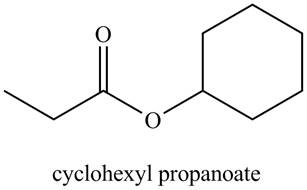
Figure 1
The structure corresponding to the given name is drawn in Figure 1.
(b)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
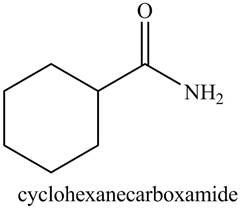
Explanation of Solution
The given name indicates that compound contains amide (name ends with -amide) as the functional group. This amide group is derived from cyclohexylcarboxylic acid, and bonded to cyclohexane ring.
Thus, the structure corresponding to given name is,
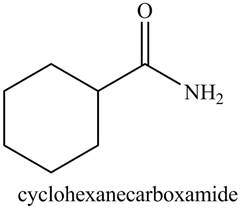
Figure 2
The structure corresponding to the given name is drawn in Figure 2.
(c)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
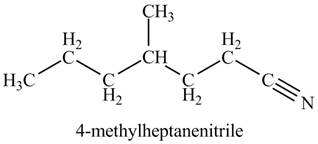
Explanation of Solution
The given name indicates that compound contains cyano (name ends with -nitrile) as the functional group, and heptane as the longest carbon chain with one methyl substitution at
Thus, the structure corresponding to given name is,
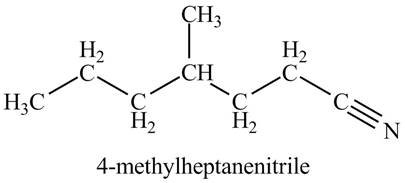
Figure 3
The structure corresponding to the given name is drawn in Figure 3.
(d)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
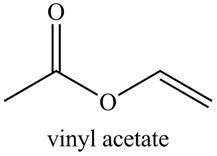
Explanation of Solution
The given name indicates that compound contains ester (name ends with -ate) as the functional group, and an acetate group is bonded to a vinyl group
Thus, the structure corresponding to the given name is,
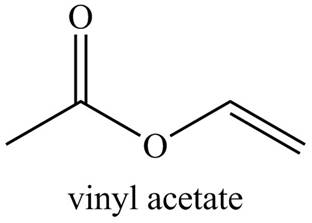
Figure 4
The structure corresponding to the given name is drawn in Figure 4.
Interpretation: The structure corresponding to the given name is to be drawn.
(e)
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
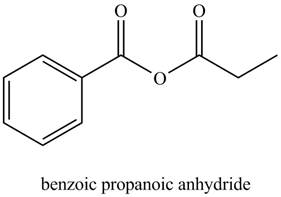
The structure corresponding to the given name is,
Explanation of Solution
The given name indicates that compound contains anhydride (name ends with -anhydride) as the functional group. This anhydride compound is derived from benzoic acid and propanoic acid.
Thus, the structure corresponding to the given name is,

Figure 5
The structure corresponding to the given name is drawn in Figure 5.
Interpretation: The structure corresponding to the given name is to be drawn.
(f)
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
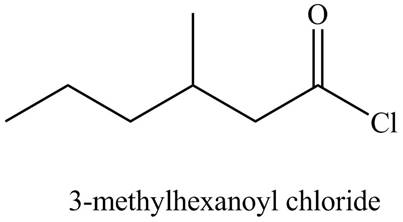
The structure corresponding to the given name is,
Explanation of Solution
The given name indicates that compound contains acid chloride (name ends with -yl chloride) as the functional group, and hexane as the longest carbon chain with one methyl substitution at
Thus, the structure corresponding to the given name is,
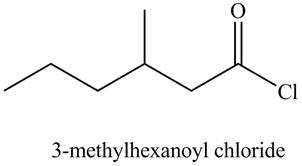
Figure 6
The structure corresponding to the given name is drawn in Figure 6.
Interpretation: The structure corresponding to the given name is to be drawn.
(g)
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
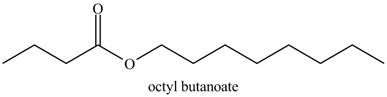
The structure corresponding to the given name is,
Explanation of Solution
The given name indicates that compound contains ester (name ends with –ate) as the functional group, and butane as the longest carbon chain with one octyl substitution as
Thus, the structure corresponding to the given name is,

Figure 7
The structure corresponding to the given name is drawn in Figure 7.
(h)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
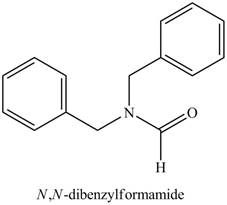
Explanation of Solution
The given name indicates that compound contains amide (name ends with -amide) as the functional group. This amide group is derived from formic acid, and bonded to two benzyl groups.
Thus, the structure corresponding to the given name is,
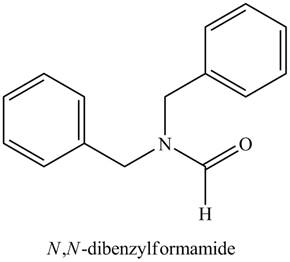
Figure 8
The structure corresponding to the given name is drawn in Figure 8.
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- a) Write out 6 completely different reactions of acetophenone (reagent, product). b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain. c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for each one. You may use preps where you just change the functional group, and/or preps where you construct the carbon chain.arrow_forward12. CH3 OH OH H&C CH3 H₂C N OH H₂C CH3 H&C CH3 H₂C' CH3 H.C CH3OH H.C CH2CH3OH CH3CEN Which one of these 17 compounds is represented by this IR and this 'H NMR spectrum? IR Spectrum 3000 4000 3000 NMR Spectrum 2000 £500 RAVENUMBER 2000 1500 9 8 6 5 10 HP-00-290 ppm m 1000 500 1000 4 °arrow_forwardDraw the structure of (E,6R) 6-methoxy-4-hepten-2-one. Give the IUPAC name of this compound, including stereochemistry. Draw the most stable chair conformation of (cis) 1,3-isobutylcyclohexane. H HC=CCH₂ CH2CH3 EN(CH3)2 -CN(CH3)2arrow_forward
- 10. Write out the mechanism (intermediate/transition state) for this reaction; indicate stereochemistry in product. H3C CH₂OH CH3 SN1 Harrow_forwardWrite "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forwardWrite "most" under the most acidic compound. Write "least" under the least acidic compound. OH NO₂ OCH3 Br 9. Compound X, C50H84F2, reacts with excess H2/Pd to give a C50H88F2 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forward
- 4. State whether these two are: a) the same molecule b) c) d) different compounds that are not isomers constitutional isomers diastereomers e) enantiomers CH3 CH₁₂ H OH HO H H OH HO H CH, CH₂ 5. a) How many stereocenters does this compound have? b) How many stereoisomers are possible for this compound? CH₂ OH CHCHarrow_forwardCalculating the pH at equivalence of a titration A chemist titrates 210.0 mL of a 0.1003 M hydrobromic acid (HBr) solution with 0.7550M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = ] ☑ o0o 18 Ararrow_forwardDo you do chemistry assignmentsarrow_forward
- Using the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A This reaction is always spontaneous, but proceeds slower at temperatures above 120. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 117. °C. AS is (pick one) ΔΗ is (pick one) This reaction is slower below 20. °C than C above. AS is |(pick one) ? 18 Ar 1arrow_forwardCalculating the pH at equivalence of a titration Try Again Your answer is incorrect. 0/5 a A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 11.43] G 00. 18 Ar B•arrow_forwardBiological Macromolecules Naming and drawing the products of aldose oxidation and reduction aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions. Click and drag to start drawing a structure. X AP ‡ 1/5 Naor Explanation Check McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





