
At point A in a Carnot cycle, 2.34 mol of a monatomic ideal gas has a pressure of 1 4000 kPa, a volume of 10.0 L, and a temperature of 720 K. The gas expands isothermally to point B and then expands adiabatically to point C, where its volume is 24.0 L. An isothermal compression brings it to point D, where its volume is 15.0 L. An adiabatic process returns the gas to point A. (a) Determine all the unknown pressures, volumes, and temperatures as you f ill in the following table:
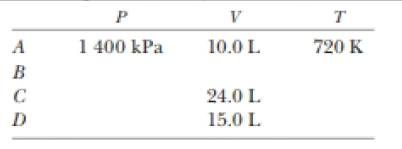
(b) Find the energy added by heat, the work done by the engine, and the change in internal energy for each of the steps A → B, B → C, C → D, and D → A (c) Calculate the efficiency Wnet/|Qk|. (d) Show that the efficiency is equal to 1 - TC/TA, the Carnot efficiency.
(a)
The unknown pressures, volumes and the temperature in the table.
Answer to Problem 22.32P
The values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation of adiabatic process
Here,
The value of
Substitute
Thus, the pressure of the gas at point
Write the ideal gas equation.
Here,
The value of gas constant is
Substitute
Thus, the temperature of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Write the equation of adiabatic process
Here,
Substitute
Thus, the volume of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Form a table and show the unknown value of pressures, volumes and temperatures.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b)
The energy added by heat, work done by the engine and the change in internal energy for each of the steps
Answer to Problem 22.32P
The values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
The process
Write the equation of change in temperature for process
Here,
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Here,
The value of
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
The process
Write the equation of change in temperature for process
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
Form a table and show the value of energy added by heat, work done by the engine and the change in internal energy.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
The value of efficiency
Answer to Problem 22.32P
The value of efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Calculate the net work done from the table is,
Write the equation for efficiency.
Here,
Substitute
The value of efficiency
Conclusion:
Therefore, the value of efficiency
(d)
To show: The efficiency is equal to the Carnot efficiency
Answer to Problem 22.32P
The efficiency is equal to the Carnot efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation for Carnot efficiency.
Here,
The value of
Substitute
Thus, the Carnot efficiency is
Write the equation for efficiency.
Substitute
The value of efficiency is
Conclusion:
Therefore, the efficiency is equal to the Carnot efficiency
Want to see more full solutions like this?
Chapter 22 Solutions
Physics for Scientists and Engineers, Volume 1
- The 10-lb weight is supported by the cord AC and roller and by the spring that has a stiffness of k = 10 lb/in. and an unstretched length of 12 in. as shown in. Part A Determine the distance d to maintain equilibrium. Express your answer in inches to three significant figures. 節 ΕΠΙ ΑΣΦ d = *k J vec 5 t 0 ? d C A in. 12 in. Barrow_forwardThe members of a truss are connected to the gusset plate as shown in . The forces are concurrent at point O. Take = 90° and T₁ = 7.5 kN. Part A Determine the magnitude of F for equilibrium. Express your answer to three significant figures and include the appropriate units. F = Value Submit Request Answer Part B 0 ? Units Determine the magnitude of T2 for equilibrium. Express your answer to three significant figures and include the appropriate units. ? T₂ = Value Units T₁ Carrow_forwardpls help on botharrow_forward
- pls helparrow_forwardpls helparrow_forward6. 6. There are 1000 turns on the primary side of a transformer and 200 turns on thesecondary side. If 440 V are supplied to the primary winding, what is the voltageinduced in the secondary winding? Is this a step-up or step-down transformer? 7. 80 V are supplied to the primary winding of a transformer that has 50 turns. If thesecondary side has 50,000 turns, what is the voltage induced on the secondary side?Is this a step-up or step-down transformer? 8. There are 50 turns on the primary side of a transformer and 500 turns on thesecondary side. The current through the primary winding is 6 A. What is the turnsratio of this transformer? What is the current, in milliamps, through the secondarywinding?9. The current through the primary winding on a transformer is 5 A. There are 1000turns on the primary winding and 20 turns on the secondary winding. What is theturns ratio of this transformer? What is the current, in amps, through the secondarywinding?arrow_forward
- No chatgpt plsarrow_forwardWhat is the current, in amps, across a conductor that has a resistance of10 Ω and a voltage of 20 V? 2. A conductor draws a current of 100 A and a resistance of 5 Ω. What is thevoltageacross the conductor? 3. What is the resistance, in ohm’s, of a conductor that has a voltage of 80 kVand acurrent of 200 mA? 4. An x-ray imaging system that draws a current of 90 A is supplied with 220V. What is the power consumed? 5. An x-ray is produced using 800 mA and 100 kV. What is the powerconsumed in kilowatts?arrow_forwardՍՈՈՒ XVirginia Western Community Coll x P Course Home X + astering.pearson.com/?courseld=13289599#/ Figure y (mm) x=0x = 0.0900 m All ✓ Correct For either the time for one full cycle is 0.040 s; this is the period. Part C - ON You are told that the two points x = 0 and x = 0.0900 m are within one wavelength of each other. If the wave is moving in the +x-direction, determine the wavelength. Express your answer to two significant figures and include the appropriate units. 0 t(s) λ = Value m 0.01 0.03 0.05 0.07 Copyright © 2025 Pearson Education Inc. All rights reserved. 日 F3 F4 F5 1775 % F6 F7 B F8 Submit Previous Answers Request Answer ? × Incorrect; Try Again; 3 attempts remaining | Terms of Use | Privacy Policy | Permissions | Contact Us | Cookie Settings 28°F Clear 4 9:23 PM 1/20/2025 F9 prt sc F10 home F11 end F12 insert delete 6 7 29 & * ( 8 9 0 t = back Οarrow_forward
- Part C Find the height yi from which the rock was launched. Express your answer in meters to three significant figures. Learning Goal: To practice Problem-Solving Strategy 4.1 for projectile motion problems. A rock thrown with speed 12.0 m/s and launch angle 30.0 ∘ (above the horizontal) travels a horizontal distance of d = 19.0 m before hitting the ground. From what height was the rock thrown? Use the value g = 9.800 m/s2 for the free-fall acceleration. PROBLEM-SOLVING STRATEGY 4.1 Projectile motion problems MODEL: Is it reasonable to ignore air resistance? If so, use the projectile motion model. VISUALIZE: Establish a coordinate system with the x-axis horizontal and the y-axis vertical. Define symbols and identify what the problem is trying to find. For a launch at angle θ, the initial velocity components are vix=v0cosθ and viy=v0sinθ. SOLVE: The acceleration is known: ax=0 and ay=−g. Thus, the problem becomes one of…arrow_forwardPhys 25arrow_forwardPhys 22arrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





