
At point A in a Carnot cycle, 2.34 mol of a monatomic ideal gas has a pressure of 1 4000 kPa, a volume of 10.0 L, and a temperature of 720 K. The gas expands isothermally to point B and then expands adiabatically to point C, where its volume is 24.0 L. An isothermal compression brings it to point D, where its volume is 15.0 L. An adiabatic process returns the gas to point A. (a) Determine all the unknown pressures, volumes, and temperatures as you f ill in the following table:
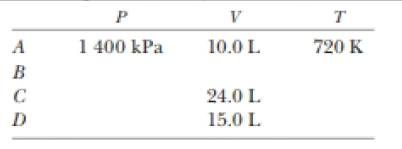
(b) Find the energy added by heat, the work done by the engine, and the change in internal energy for each of the steps A → B, B → C, C → D, and D → A (c) Calculate the efficiency Wnet/|Qk|. (d) Show that the efficiency is equal to 1 - TC/TA, the Carnot efficiency.
(a)
The unknown pressures, volumes and the temperature in the table.
Answer to Problem 22.32P
The values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation of adiabatic process
Here,
The value of
Substitute
Thus, the pressure of the gas at point
Write the ideal gas equation.
Here,
The value of gas constant is
Substitute
Thus, the temperature of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Write the equation of adiabatic process
Here,
Substitute
Thus, the volume of the gas at point
In isothermal process, the temperature is constant.
For isothermal process
The temperature of the gas at point
Thus, the temperature of the gas at point
Write the ideal gas equation.
Here,
Substitute
Thus, the pressure of the gas at point
Form a table and show the unknown value of pressures, volumes and temperatures.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of unknown pressures, volumes and the temperature in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b)
The energy added by heat, work done by the engine and the change in internal energy for each of the steps
Answer to Problem 22.32P
The values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
The process
Write the equation of change in temperature for process
Here,
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Here,
The value of
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
The process
Write the equation of change in temperature for process
Substitute
Thus, the change in internal energy for process
Write the equation of work done by the engine for process
Substitute
Thus, the work done by the engine for process
Write the equation of isothermal process
Substitute
Thus, the energy added by heat for process
Write the equation of change in temperature for process
Substitute
Substitute
Thus, the change in internal energy for process
The process
Thus, the energy added by heat for process
Write the equation of change in internal energy for process
Substitute
Thus, the work done by the engine for process
Form a table and show the value of energy added by heat, work done by the engine and the change in internal energy.
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conclusion:
Therefore, the values of energy added by heat, work done by the engine and the change in internal energy for each of the steps in the table are,
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(c)
The value of efficiency
Answer to Problem 22.32P
The value of efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Calculate the net work done from the table is,
Write the equation for efficiency.
Here,
Substitute
The value of efficiency
Conclusion:
Therefore, the value of efficiency
(d)
To show: The efficiency is equal to the Carnot efficiency
Answer to Problem 22.32P
The efficiency is equal to the Carnot efficiency
Explanation of Solution
Given: The number of moles of a mono atomic ideal gas is
Write the equation for Carnot efficiency.
Here,
The value of
Substitute
Thus, the Carnot efficiency is
Write the equation for efficiency.
Substitute
The value of efficiency is
Conclusion:
Therefore, the efficiency is equal to the Carnot efficiency
Want to see more full solutions like this?
Chapter 22 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
- A hydrogen atom has just a single electron orbiting the nucleus, which happens to be a single proton without any neutrons. The proton is positively charged, the electron negatively, but both with the same magnitude of charge given by e=1.602x10-19C. The mass of an electron is 9.11x10-31kg, and the proton is 1.67x10-27kg. Find the ratio of the electrostatic to the gravitational force of attraction between the electron and the proton in hydrogen. \arrow_forwardWhat is the third law pair to the normal force as you sit in a chair? What effect does the sun's pull on earth have in terms of third law pairs?arrow_forwardUsing Newton's 2nd law, show that all objects subject to the pull of gravity alone should fall at the same rate. What is that rate?arrow_forward
- No chatgpt pls will upvotearrow_forwardA cart on wheels (assume frictionless) with a mass of 20 kg is pulled rightward with a 50N force. What is its acceleration?arrow_forwardLight travels through a vacuum at a speed of 2.998 x 108m/s. Determine the speed of light in the following media: crown glass (n = 1.52)arrow_forward
- 2.62 Collision. The engineer of a passenger train traveling at 25.0 m/s sights a freight train whose caboose is 200 m ahead on the same track (Fig. P2.62). The freight train is traveling at 15.0 m/s in the same direction as the passenger train. The engineer of the passenger train immediately applies the brakes, causing a constant acceleration of 0.100 m/s² in a direction opposite to the train's velocity, while the freight train continues with constant speed. Take x = 0 at the location of the front of the passenger train when the engineer applies the brakes. (a) Will the cows nearby witness a collision? (b) If so, where will it take place? (c) On a single graph, sketch the positions of the front of the pas- senger train and the back of the freight train.arrow_forwardCan I get help with how to calculate total displacement? The answer is 78.3x-4.8yarrow_forward2.70 Egg Drop. You are on the Figure P2.70 roof of the physics building, 46.0 m above the ground (Fig. P2.70). Your physics professor, who is 1.80 m tall, is walking alongside the building at a constant speed of 1.20 m/s. If you wish to drop an egg on your profes- sor's head, where should the profes- sor be when you release the egg? Assume that the egg is in free fall. 2.71 CALC The acceleration of a particle is given by ax(t) = -2.00 m/s² +(3.00 m/s³)t. (a) Find the initial velocity Vox such that v = 1.20 m/s 1.80 m 46.0 marrow_forward
- One has to push down a ball with a force of 470 Newtons in order to hold the ball still, completely submerged under the surface of the water. What is the volume of the styrofoam ball in cubic meters? Use 997 kg/m3 as the density of water, 95 kg/m3 for the density of the styrofoam, and g = 9.8 m/s2.arrow_forwardThe cube is placed in a bucket of water and find that it floats, with 33% of its volume submerged below the surface of the water. What is the density of the mystery material? The material is uniformly distributed throughout the solid cube, with the number of kg/m3.arrow_forward2.82 A ball is thrown straight up from the ground with speed Up. At the same instant, a second ball is dropped from rest from a height H, directly above the point where the first ball was thrown upward. There is no air resistance. (a) Find the time at which the two balls collide. (b) Find the value of H in terms of un, and g such that at the instant when the balls collide, the first ball is at the highest point of its motion.arrow_forward
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





