
EBK ORGANIC CHEMISTRY
9th Edition
ISBN: 8220100591310
Author: McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.SE, Problem 73AP
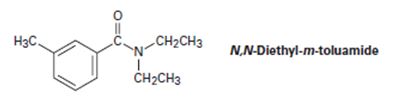
N,N-Diethyl-m-toluamide (DEET) is the active ingredient in many insect-repellent preparations. How might you synthesize this substance from m-bromotoluene?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
4. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton
transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted
without ambiguity.
a)
NHBoc
⚫OBn
HO.
H3C
CO2CH3
-OBn
H3C
H3C.
H3C.
NHBOC
CI
CO2CH3
Draw structures of the following compounds and identify their role:
mCPBA
(MCPBA)
DMS
Py
9-BBN
LAH
Sia₂BH
TsCI
PCC
t-BuOK
LDA
MeLi
n-BuLi
DMSO
DMF
Sodium Borohydride
Lithium DiisopropylAmide
2
Using Luther's rule, calculate the reference potential of the Hg2+/Hg redox electrode.
DATA: Electrode potentials E° = 0,854 V y E 0,788 V
Hg2+/Hg
2+
Hg2/Hg
Chapter 21 Solutions
EBK ORGANIC CHEMISTRY
Ch. 21.1 - Give IUPAC names for the following substances:Ch. 21.1 - Draw structures corresponding to the following...Ch. 21.2 - Prob. 3PCh. 21.2 - Rank the compounds in each of the following sets...Ch. 21.2 - Predict the products of the following nucleophilic...Ch. 21.2 - Prob. 6PCh. 21.3 - Prob. 7PCh. 21.3 - If the following molecule is treated with acid...Ch. 21.4 - How might you prepare the following esters using a...Ch. 21.4 - Prob. 10P
Ch. 21.4 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - What product would you expect from reaction of one...Ch. 21.6 - Prob. 16PCh. 21.6 - Prob. 17PCh. 21.6 - Show the products you would obtain by reduction of...Ch. 21.6 - What ester and what Grignard reagent might you...Ch. 21.7 - Prob. 20PCh. 21.7 - How would you use the reaction of an amide with...Ch. 21.8 - Write the mechanism of the reaction shown in...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.10 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.SE - Name the following compounds:Ch. 21.SE - Prob. 28VCCh. 21.SE - Prob. 29VCCh. 21.SE - Prob. 30VCCh. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the complete...Ch. 21.SE - Prob. 35MPCh. 21.SE - When 4-dimethylaminopyridine (DMAP) is added in...Ch. 21.SE - Prob. 37MPCh. 21.SE - Prob. 38MPCh. 21.SE - Prob. 39MPCh. 21.SE - The hydrolysis of a biological thioester to the...Ch. 21.SE - Prob. 41MPCh. 21.SE - Prob. 42MPCh. 21.SE - Prob. 43MPCh. 21.SE - In the iodoform reaction, a triiodomethyl ketone...Ch. 21.SE - Give IUPAC names for the following compounds:Ch. 21.SE - Prob. 46APCh. 21.SE - Draw and name compounds that meet the following...Ch. 21.SE - Predict the product, if any, of reaction between...Ch. 21.SE - Prob. 49APCh. 21.SE - Prob. 50APCh. 21.SE - What product would you expect to obtain from...Ch. 21.SE - Prob. 52APCh. 21.SE - Prob. 53APCh. 21.SE - The following reactivity order has been found for...Ch. 21.SE - Prob. 55APCh. 21.SE - Outline methods for the preparation of...Ch. 21.SE - Prob. 57APCh. 21.SE - When ethyl benzoate is heated in methanol...Ch. 21.SE - tert-Butoxycarbonyl azide, a reagent used in...Ch. 21.SE - Prob. 60APCh. 21.SE - Prob. 61APCh. 21.SE - What is the structure of the polymer produced by...Ch. 21.SE - Polyimides with the structure shown are used as...Ch. 21.SE - Prob. 64APCh. 21.SE - Propose a structure for a compound, C4H7ClO2, that...Ch. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Prob. 67APCh. 21.SE - When a carboxylic acid is dissolved in...Ch. 21.SE - Prob. 69APCh. 21.SE - Prob. 70APCh. 21.SE - Prob. 71APCh. 21.SE - Phenyl 4-aminosalicylate is a drug used in the...Ch. 21.SE - N,N-Diethyl-m-toluamide (DEET) is the active...Ch. 21.SE - Tranexamic acid, a drug useful against blood...Ch. 21.SE - One frequently used method for preparing methyl...Ch. 21.SE - Prob. 76APCh. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Propose structures for compounds with the...Ch. 21.SE - Propose a structure for the compound with the...Ch. 21.SE - Draw the structure of the compound that produced...Ch. 21.SE - Prob. 81APCh. 21.SE - Epoxy adhesives are prepared in two steps. SN2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following compound: HO -H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: H-O: H-OH H-O HH H-OO Mechanism B: H-Q Mechanism C: Θ OH H-O: Mechanism D: H-O H- H-OO C H-OO H- H- H-OO HH OH -H - HON H :OH H-Harrow_forwardidentify the product (or multiple products) for each of the following reactions: CI 1) NaNH2 (excess) ठ Cl 2) H₂O Hz H₂SO₂, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) 1)9-BBN 2) H₂O, NaOH ? Br H A B C afó gó H OA B O c OD E OF D E F H H Na, NHarrow_forwardIdentify the product (or multiple products) for each of the following reactions: ? or CI CI 1) NaNHz (excess) 2) H₂O OA OB O C OD OE OF H₂SO₂, H₂O Hq50. 1) n-BuLi 2) Br 1) 9-BBN 2) H₂O₂, NaOH A B H H متته D E H H H H C H H F H H H₂ Lindlar's catalyst Na NHarrow_forward
- Identify the product (or multiple products) for each of the following reactions: O A OB Oc OD OE OF CI CI 1) NaNH2 (excess) 2) H₂O H₂ H₂SO2, H₂O HgSO Lindlar's catalyst 1) n-BuLi 2) Br 1)9-BBN 2) H₂O₂, NaOH ? Na, NH3 C H A H H مننه مننه منن مننه H F H H E مند H D H Harrow_forwardFor the following compound: HO H Draw a mechanism for the tautomerization process under BASIC conditions: Mechanism A: + H-O: H-OH₂ H Mechanism B: H-Ö: HO-H H-OO -H H HH H H HH H-O: H-OO H-OO -H H e -H : OH Θ Mechanism C: Θ A : OH H-O: H H H-O-H 0. Mechanism D: e.. : OH :0 H H-O-H H-O: H-OO :O H -H H H сём H 0 :0 + H Θ H H H-arrow_forwardFor the following compound: H OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Θ :OH O O-H HO 0: Mechanism B: :O-H e.. Θ :OH Mechanism C: H HO-H :0: Θ 0: H H e.. : OH 0: "Θ HH O. :OH :OH O-H O-H Mechanism D: :OH H-OH₂ :OH HO-H 0: © O-H H HH 0: HHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY