
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.1B, Problem 2.3P
For each of the following compounds
- 1. Draw the Lewis structure.
- 2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment.
- 3. Estimate whether the compound will have a large small, or zero dipole moment.
- a. NH4+
- b. O3
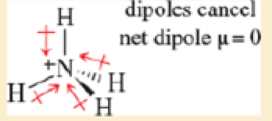
- a. NH4+ has four polar N—H bonds These bonds are probably more polarized than a typical N—H bond, because the N in NH4+ bears a formal positive charge Nevertheless, these four colar bonds have a symmetric tetrahedral arrangement so they cancel each other.
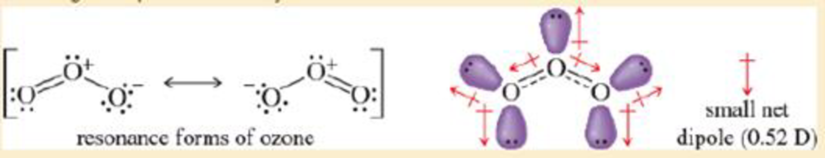
- b. Ozone (O3) is an sp2 hybrid structure with a lone pair on the central oxygen atom Therefore O3 must be bent. The resonance structures imply partia negatve charges on the outer oxygens and a partial positive charge on the central oxygen. The lone pair on the central oxygen cancels part, but not all, of the vector sum of the two O—O dipoles The resulting not d polo is relatively small.
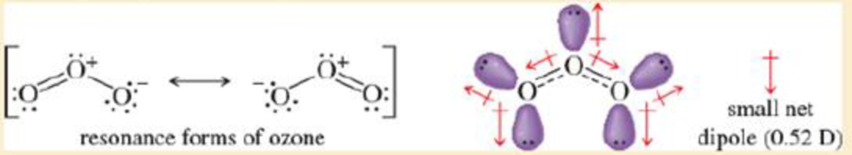
- a. Ozone (O3) is an sp2 hybrid structure, with a lone pair on the central oxygon atom Therefore, O3 must be bent. The resonance structures imply partial negative charges on the outer oxygens end a partial positive charge on the central oxygen. The lone pair on the central oxygen cancels part but not all, of the vector sum of the two O—O dipoles The resulting net dipole is relatively small.
- b. CH2CI2
- c. CH3F
- d. CF4
- e. CH3OH
- f. HCN
- g. CH3CHO
- h. H2C=NH
- i. (CH3)3N
- j. CH2=CHCI
- k. BF3
- l. BeCl2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Topic: Photochemistry and Photophysics of Supramolecules
Two cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 C
With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.
Chapter 2 Solutions
Organic Chemistry (9th Edition)
Ch. 2.1A - Prob. 2.1PCh. 2.1B - The NF bond is more polar than the NH bond: but...Ch. 2.1B - For each of the following compounds 1. Draw the...Ch. 2.1B - Two isomers of 1,2-dichloroethene are known One...Ch. 2.2C - Prob. 2.5PCh. 2.2C - Prob. 2.6PCh. 2.3 - Prob. 2.7PCh. 2.4 - Calculate the pH of the following solutions a....Ch. 2.6A - Ammonia appears in Table 2-2 as both an acid and a...Ch. 2.7 - Write equations for the following acid-base...
Ch. 2.7 - Ethanol, methylamine. and acetic acid are all...Ch. 2.8 - Prob. 2.12PCh. 2.10 - Write equations for the following acid-base...Ch. 2.10 - Rank the following acids in decreasing order of...Ch. 2.11 - Prob. 2.15PCh. 2.11 - Prob. 2.16PCh. 2.11 - Consider each pair of bases and explain which one...Ch. 2.12 - Which is a stronger base ethoxide ion or acetate...Ch. 2.12 - Prob. 2.19PCh. 2.12 - Prob. 2.20PCh. 2.12 - Prob. 2.21PCh. 2.12 - Choose the more basic member of each pair of...Ch. 2.14 - Prob. 2.23PCh. 2.15D - Classify the following hydrocarbons and draw a...Ch. 2.16D - Prob. 2.25PCh. 2.17C - Draw a Lewis structure and classify each of the...Ch. 2.17C - Circle the functional groups in the following...Ch. 2 - The CN triple bond in acetonitrile has a dipole...Ch. 2 - Prob. 2.29SPCh. 2 - Sulfur dioxide has a dipole moment of 1.60 D....Ch. 2 - Which of the following pure compounds can form...Ch. 2 - Predict which member of each pair is more soluble...Ch. 2 - Prob. 2.33SPCh. 2 - Prob. 2.34SPCh. 2 - Predict which compound in each pair has the higher...Ch. 2 - All of the following compounds can react as acids...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - The Ka of phenylacetic acid is 5 2 105, and the...Ch. 2 - The following compound can become protonated on...Ch. 2 - The following compounds are listed in increasing...Ch. 2 - Prob. 2.42SPCh. 2 - Prob. 2.43SPCh. 2 - Compare the relative acidity of 1-molar aqueous...Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - The following compounds can all react as bases. a....Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - Prob. 2.48SPCh. 2 - Methyllithium (CH3Li) is often used as a base in...Ch. 2 - Label the reactants in these acid-base reactions...Ch. 2 - In each reaction, label the reactants as Lewis...Ch. 2 - Prob. 2.52SPCh. 2 - Each of these compounds can react as a nucleophile...Ch. 2 - Prob. 2.54SPCh. 2 - Give a definition and an example for each class of...Ch. 2 - Circle the functional groups in the following...Ch. 2 - Prob. 2.57SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY