
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
4th Edition
ISBN: 9781260269284
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 71P
What class of enzyme catalyzes each of the following reactions?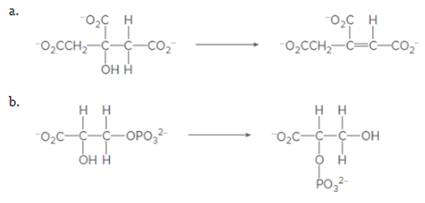
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 21 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
Ch. 21.2 - In addition to the amino and carboxyl groups, what...Ch. 21.2 - Draw both enantiomers of each amino acid in...Ch. 21.2 - Which of the following amino acids is naturally...Ch. 21.3 - Draw the structure of the amino acid valine at...Ch. 21.3 - Identify the amino acid shown with all uncharged...Ch. 21.4 - Identify the N-terminal and C-terminal amino acid...Ch. 21.4 - Prob. 21.4PCh. 21.4 - Prob. 21.4PPCh. 21.4 - Prob. 21.5PCh. 21.4 - Prob. 21.5PP
Ch. 21.4 - Prob. 21.6PPCh. 21.5 - Prob. 21.6PCh. 21.6 - Prob. 21.7PCh. 21.6 - Prob. 21.8PCh. 21.6 - Prob. 21.9PCh. 21.7 - Why is hemoglobin more water soluble than ...Ch. 21.8 - Prob. 21.7PPCh. 21.8 - Prob. 21.11PCh. 21.9 - Prob. 21.8PPCh. 21.9 - Prob. 21.12PCh. 21.9 - Prob. 21.9PPCh. 21.9 - Prob. 21.13PCh. 21.10 - Prob. 21.14PCh. 21.10 - Prob. 21.15PCh. 21.10 - Prob. 21.16PCh. 21.10 - Prob. 21.17PCh. 21.10 - The nerve gas sarin acts as a poison by covalently...Ch. 21.10 - Prob. 21.19PCh. 21.10 - Explain why the proteins involved in blood...Ch. 21 - The amino acid alanine is a solid at room...Ch. 21 - Why is phenylalanine water soluble but...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - Draw the structure of a naturally occurring amino...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - For each amino acid: [1] draw the L enantiomer in...Ch. 21 - Draw both enantiomers of each amino acid and label...Ch. 21 - Which of the following Fischer projections...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - For each amino acid: [1] give the name; [2] give...Ch. 21 - (a) Identify the amino acid shown with all...Ch. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Draw the structure of the neutral, positively...Ch. 21 - Locate the peptide bond in the dipeptide shown in...Ch. 21 - Label the N-terminal and C-terminal amino acids in...Ch. 21 - Melittin, the principal toxin of bee venom,...Ch. 21 - Cobratoxin is a neurotoxin found in the venom of...Ch. 21 - (a) Draw the structure of the two possible...Ch. 21 - (a) Draw the structure of the two possible...Ch. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - For each tripeptide: [1] draw the structure of the...Ch. 21 - Prob. 43PCh. 21 - For each tripeptide: [1] identify the amino acids...Ch. 21 - What amino acids are formed by hydrolysis of the...Ch. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - Draw the structures of the amino acids formed when...Ch. 21 - Prob. 49PCh. 21 - Prob. 50PCh. 21 - Prob. 51PCh. 21 - Trypsin is a digestive enzyme that hydrolyzes...Ch. 21 - What type of intermolecular forces exist between...Ch. 21 - What type of interaction occur at each of the...Ch. 21 - Which peptide in each pair contains amino acids...Ch. 21 - Decide if the side chains of the amino acid...Ch. 21 - Which type of protein structure is indicated in...Ch. 21 - Label each of the following diagrams as...Ch. 21 - Prob. 59PCh. 21 - Prob. 60PCh. 21 - Prob. 61PCh. 21 - Prob. 62PCh. 21 - Compare - keratin and hemoglobin with regard to...Ch. 21 - Compare collagen and myoglobin with regard to each...Ch. 21 - Prob. 65PCh. 21 - Prob. 66PCh. 21 - Describe the function or biological activity of...Ch. 21 - Describe the function or biological activity of...Ch. 21 - Prob. 69PCh. 21 - Prob. 70PCh. 21 - What class of enzyme catalyzes each of the...Ch. 21 - What class of enzyme catalyzes each of the...Ch. 21 - Prob. 73PCh. 21 - Prob. 74PCh. 21 - Prob. 75PCh. 21 - What kind of reaction is catalyzed by each of the...Ch. 21 - Prob. 77PCh. 21 - How will each of the following changes affect the...Ch. 21 - Prob. 79PCh. 21 - Prob. 80PCh. 21 - Prob. 81PCh. 21 - Prob. 82PCh. 21 - Prob. 83PCh. 21 - Prob. 84PCh. 21 - Prob. 85PCh. 21 - Prob. 86PCh. 21 - Why must vegetarian diets be carefully balanced?Ch. 21 - Prob. 88PCh. 21 - Sometimes an incision is cauterized (burned) to...Ch. 21 - Why is insulin administered by injection instead...Ch. 21 - Prob. 91PCh. 21 - The silk produced by a silkworm is a protein with...Ch. 21 - Explain the difference in the mechanism of action...Ch. 21 - Prob. 94PCh. 21 - Prob. 95CPCh. 21 - Suggest a reason for the following observation....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY