
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
4th Edition
ISBN: 9780134110684
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 6CQ
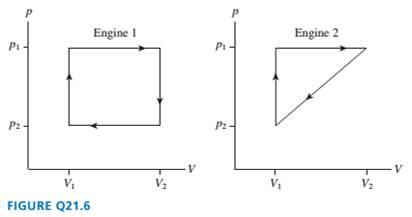
FIGURE Q21.6 shows the
engines. Which
are they the same? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
As a ball falls under the influence of gravity, does gravity do positive work or negative work? Provide a mathematical explanation.
Under what circumstances is it bad to describe kinetic energy as k = 1/2mv^2
No chatgpt pls will upvote
Chapter 21 Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Ch. 21 - Prob. 1CQCh. 21 - Rank in order, from largest to smallest, the...Ch. 21 - Prob. 3CQCh. 21 - FIGURE Q21.4 shows the pV diagram of a heat...Ch. 21 - Rank in order, from largest to smallest, the...Ch. 21 - FIGURE Q21.6 shows the thermodynamic cycles of two...Ch. 21 - A heat engine satisfies Wout= Qnet. Why is there...Ch. 21 - Prob. 8CQCh. 21 - Prob. 9CQCh. 21 - Prob. 10CQ
Ch. 21 - Prob. 11CQCh. 21 - Prob. 1EAPCh. 21 - Prob. 2EAPCh. 21 - Prob. 3EAPCh. 21 - Prob. 4EAPCh. 21 - Prob. 5EAPCh. 21 - Prob. 6EAPCh. 21 - The power output of a car engine running at 2400...Ch. 21 - Prob. 8EAPCh. 21 - Prob. 9EAPCh. 21 - Prob. 10EAPCh. 21 - Prob. 11EAPCh. 21 - Prob. 12EAPCh. 21 - Prob. 13EAPCh. 21 - Prob. 14EAPCh. 21 - Prob. 15EAPCh. 21 - Prob. 16EAPCh. 21 - A heat engine uses a diatomic gas in a Brayton...Ch. 21 - At what pressure ratio does a Brayton cycle using...Ch. 21 - Prob. 19EAPCh. 21 - Prob. 20EAPCh. 21 - Prob. 21EAPCh. 21 - Prob. 22EAPCh. 21 - Prob. 23EAPCh. 21 - Prob. 24EAPCh. 21 - Prob. 25EAPCh. 21 - Prob. 26EAPCh. 21 - Prob. 27EAPCh. 21 - A Carnot engine whose hot-reservoir temperature is...Ch. 21 - Prob. 29EAPCh. 21 - A heat engine operating between energy reservoirs...Ch. 21 - Prob. 31EAPCh. 21 - A Carnot refrigerator operating between —20°C and...Ch. 21 - The coefficient of performance of a refrigerator...Ch. 21 - A Carnot heat engine with thermal efficiency 1/3...Ch. 21 - Prob. 35EAPCh. 21 - Prob. 36EAPCh. 21 - A heat engine with 50% of the Carnot efficiency...Ch. 21 - Prove that the work done in an adiabatic process i...Ch. 21 - Prob. 39EAPCh. 21 - Prob. 40EAPCh. 21 - An ideal refrigerator utilizes a Carnot cycle...Ch. 21 - Prob. 42EAPCh. 21 - There has long been an interest in using the vast...Ch. 21 - A Carnot heat engine operates between reservoirs...Ch. 21 - A Carnot engine operates between temperatures of...Ch. 21 - Prob. 46EAPCh. 21 - A Carnot heat engine and an ordinary refrigerator...Ch. 21 - 48. A heat engine running backward is called a...Ch. 21 - 49. A car's internal combustion engine can be...Ch. 21 - Prob. 50EAPCh. 21 - Prob. 51EAPCh. 21 - Prob. 52EAPCh. 21 - Prob. 53EAPCh. 21 - Prob. 54EAPCh. 21 - Prob. 55EAPCh. 21 - Prob. 56EAPCh. 21 - Prob. 57EAPCh. 21 - A heat engine using a monatomic gas follows the...Ch. 21 - Prob. 59EAPCh. 21 - Prob. 60EAPCh. 21 - Prob. 61EAPCh. 21 - Prob. 62EAPCh. 21 - Prob. 63EAPCh. 21 - Prob. 64EAPCh. 21 - Prob. 65EAPCh. 21 - Prob. 66EAPCh. 21 - Prob. 67EAPCh. 21 - Prob. 68EAPCh. 21 - Prob. 69EAPCh. 21 - Prob. 70EAPCh. 21 - A refrigerator using helium gas operates on the...Ch. 21 - Prob. 72EAPCh. 21 - The gasoline engine in your car can be modeled as...Ch. 21 - Prob. 74EAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Air temperature of 37 °C increases swimming pool temperature of 2.55 °C. What is the fraction of the water in the pool must evaporate during this time to carry enough energy to keep the temperature of the pool constant? 4186 J/(kg°C) = specific heat of water 2,430,000 (2.43 x 106) J/kg = latent heat of vaporization for the water in the pool.arrow_forwardThe iceberg requires 7.4 x 1020 Joules of energy to melt it completely. It absorbs energy from the Sun at a constant average rate of 88 Watts/m2. The total surface area of iceberg exposed to the sunlight is 12 billion (1.2 x 1010) square meters. How long will it take for sunlight to melt the entire iceberg in yearsarrow_forward1.0 kg block of ice to melt in the kitchen. The temperature in the kitchen is 31 °C. The ice starts out at 0 °C and takes an hour to melt and reach the same temperature as the surrounding room (31 °C). How much heat does the 1.0 kg of ice/water absorb from the room as it melts and heats up to 31 °C in Joules absorbed? Latent heat of fusion for water/ice is 334,000 J/kg Specific heat of water is 4186 J/kg°Carrow_forward
- 5.84 If the coefficient of static friction between a table and a uni- form, massive rope is μ, what fraction of the rope can hang over the edge of the table without the rope sliding? 5.97 Block A, with weight Figure P5.97 3w, slides down an inclined plane S of slope angle 36.9° at a constant speed while plank B, with weight w, rests on top of A. The plank is attached by a cord to the wall (Fig. P5.97). (a) Draw a diagram of all the forces acting on block A. (b) If the coefficient of kinetic friction is the same between A and B and between S and A, determine its value. 36.9° 1arrow_forwardNo chatgpt pls will upvotearrow_forwardPlease solve and answer the problem correctly please. Thank you!!arrow_forward
- Please solve and answer the problem correctly please. Thank you!!arrow_forwardPlease solve all the questions correctly please. Thank you!!arrow_forwardPlease solve this problem correctly please and be sure to provide explanation on each step so I can understand what's been done thank you. (preferrably type out everything)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY