
HUMAN BIOLOGY
16th Edition
ISBN: 9781260233032
Author: Mader
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2.1, Problem 4LO
Summary Introduction
To distinguish:
In-between ionic and covalent bonds.
Introduction:
The atoms like sodium and chloride form ionic bond when the electrons in the outer shell give and take electrons to achieve a stable state of eight electrons in the valence shell of the atom. The sodium atom in an ionized state has a positive charge and the chlorine atom in an ionized state has a negative charge. The covalent bond is formed by the sharing of the electrons in the outer shell orbital of the atoms. The electrons are stable in this state.
Pictorial representation:
The ionic and covalent bonding is well represented in the Fig.1.
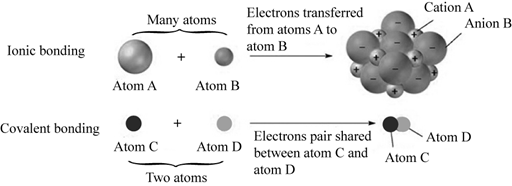
Fig.1: Ionic bonding and Covalent bonding
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is behavioral adapt
22. Which of the following mutant proteins is expected to have a dominant negative effect when over-
expressed in normal cells?
a. mutant PI3-kinase that lacks the SH2 domain but retains the kinase function
b. mutant Grb2 protein that cannot bind to RTK
c. mutant RTK that lacks the extracellular domain
d. mutant PDK that has the PH domain but lost the kinase function
e. all of the above
What is the label ?
Chapter 2 Solutions
HUMAN BIOLOGY
Ch. 2.1 - Prob. 1LOCh. 2.1 - 2. Describe the structure of an atom.
Ch. 2.1 - Prob. 3LOCh. 2.1 - 4. Distinguish between ionic and covalent bonds.
Ch. 2.1 - List the number of electrons, neutrons, and...Ch. 2.1 - Prob. 2CYPCh. 2.1 - Explain the beneficial uses of radioisotopes.Ch. 2.1 - Summarize the differences between ionic and...Ch. 2.2 - Describe the properties of water.Ch. 2.2 - Prob. 2LO
Ch. 2.2 - Prob. 3LOCh. 2.2 - List the characteristics of water and explain how...Ch. 2.2 - Prob. 2CYPCh. 2.2 - Prob. 3CYPCh. 2.3 - List the four classes of organic molecules that...Ch. 2.3 - Describe the processes by which the organic...Ch. 2.3 - Prob. 1CYPCh. 2.3 - List the four classes of organic molecules.Ch. 2.3 - Prob. 3CYPCh. 2.4 - Prob. 1LOCh. 2.4 - Prob. 2LOCh. 2.4 - Prob. 3LOCh. 2.4 - Prob. 4LOCh. 2.4 - Prob. 1BTHCh. 2.4 - Prob. 2BTHCh. 2.4 - Explain the differences between a monosaccharide,...Ch. 2.4 - Prob. 2CYPCh. 2.4 - Prob. 3CYPCh. 2.5 - Prob. 1LOCh. 2.5 - Prob. 2LOCh. 2.5 - Why might physicians resort to calling HDLs and...Ch. 2.5 - Prob. 1.2BTHCh. 2.5 - Prob. 2.1BTHCh. 2.5 - Prob. 2.2BTHCh. 2.5 - Prob. 1CYPCh. 2.5 - Prob. 2CYPCh. 2.5 - Prob. 3CYPCh. 2.6 - Prob. 1LOCh. 2.6 - Prob. 2LOCh. 2.6 - List the four levels of protein structure and...Ch. 2.6 - Prob. 1CYPCh. 2.6 - Prob. 2CYPCh. 2.6 - Prob. 3CYPCh. 2.7 - Prob. 1LOCh. 2.7 - Prob. 2LOCh. 2.7 - Prob. 1CYPCh. 2.7 - Prob. 2CYPCh. 2.7 - Prob. 3CYPCh. 2 - Prob. 1ACh. 2 - Prob. 2ACh. 2 - Prob. 3ACh. 2 - Prob. 4ACh. 2 - Prob. 5ACh. 2 - Prob. 6ACh. 2 - Prob. 7ACh. 2 - Prob. 8ACh. 2 - Prob. 9ACh. 2 - Prob. 10ACh. 2 - Prob. 11ACh. 2 - Prob. 12ACh. 2 - Prob. 13ACh. 2 - Prob. 14ACh. 2 - Prob. 15ACh. 2 - Prob. 16ACh. 2 - Prob. 17ACh. 2 - Prob. 18ACh. 2 - Prob. 19ACh. 2 - This nucleic acid is typically involved in energy...Ch. 2 - Prob. 1TCCh. 2 - Prob. 2TCCh. 2 - Name another substance that is needed in the body...Ch. 2 - Prob. 4TCCh. 2 - Prob. 5TC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Can you described the image? Can you explain the question as well their answer and how to get to an answer to an problem like this?arrow_forwardglg 112 mid unit assignment Identifying melting processesarrow_forwardGive only the mode of inheritance consistent with all three pedigrees and only two reasons that support this, nothing more, (it shouldn't take too long)arrow_forward
- Oarrow_forwardDescribe the principle of homeostasis.arrow_forwardExplain how the hormones of the glands listed below travel around the body to target organs and tissues : Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forward
- What are the functions of the hormones produced in the glands listed below: Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forwardDescribe the hormones produced in the glands listed below: Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forwardPlease help me calculate drug dosage from the following information: Patient weight: 35 pounds, so 15.9 kilograms (got this by dividing 35 pounds by 2.2 kilograms) Drug dose: 0.05mg/kg Drug concentration: 2mg/mLarrow_forward
- A 25-year-old woman presents to the emergency department with a 2-day history of fever, chills, severe headache, and confusion. She recently returned from a trip to sub-Saharan Africa, where she did not take malaria prophylaxis. On examination, she is febrile (39.8°C/103.6°F) and hypotensive. Laboratory studies reveal hemoglobin of 8.0 g/dL, platelet count of 50,000/μL, and evidence of hemoglobinuria. A peripheral blood smear shows ring forms and banana-shaped gametocytes. Which of the following Plasmodium species is most likely responsible for her severe symptoms? A. Plasmodium vivax B. Plasmodium ovale C. Plasmodium malariae D. Plasmodium falciparumarrow_forwardStandard Concentration (caffeine) mg/L Absorbance Reading 10 0.322 20 0.697 40 1.535 60 2.520 80 3.100arrow_forwardPlease draw in the missing answer, thank youarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license